Epoxymicranthols A—N, 5,9-Epoxygrayanane Diterpenoids as Potent Analgesics from Rhododendron micranthum
Pengfei Jin
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030 China
Search for more papers by this authorXinghua Yuan
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030 China
Search for more papers by this authorXiaomin Ma
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030 China
Search for more papers by this authorGuijuan Zheng
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030 China
Search for more papers by this authorRu Wang
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030 China
Search for more papers by this authorNa Sun
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030 China
Search for more papers by this authorCorresponding Author
Guangmin Yao
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030 China
E-mail: [email protected]Search for more papers by this authorPengfei Jin
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030 China
Search for more papers by this authorXinghua Yuan
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030 China
Search for more papers by this authorXiaomin Ma
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030 China
Search for more papers by this authorGuijuan Zheng
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030 China
Search for more papers by this authorRu Wang
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030 China
Search for more papers by this authorNa Sun
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030 China
Search for more papers by this authorCorresponding Author
Guangmin Yao
Hubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030 China
E-mail: [email protected]Search for more papers by this authorMain observation and conclusion
Fifteen 5,9-epoxygrayanane diterpenoids (1—15) including fourteen new ones, epoxymicranthols A—N (1—14), were isolated from the leaves extract of Rhododendron micranthum. Their structures were elucidated via extensive spectroscopic methods and 13C NMR-DP4+ analysis, and the absolute configurations of 1, 3—10, 14, and 15 were confirmed by single-crystal X-ray diffraction analysis. Epoxymicranthols A—C (1—3) represent the first examples of 14α-hydroxygrayanane diterpenoids, and epoxymicranthols C—F (3—6) are the first examples of 10β-hydroxy-5,9-epoxygrayanane diterpenoids. Meanwhile, epoxymicranthols K (11) and N (14) represent the first examples of 13-hydroxy-5,9-epoxygrayanane and 5,9-epoxygrayan-3-one diterpenoids, respectively. All the diterpenoids (1—15) were assayed for their analgesic activities, and all of them exhibited significant analgesic activities at a dose of 5.0 mg/kg. Among them, compounds 2, 7, 8, and 10 also exhibited significant analgesic activities even at lower doses of 1.0 and 0.2 mg/kg. More importantly, epoxymicranthol G (7) still expressed potent analgesic activity at a lower dose of 0.04 mg/kg. A preliminary structure-activity relationship for the analgesic effects of 5,9-epoxygrayanane diterpenoids 1—15 is discussed. These results not only enlarged the structural diversity of 5,9-epoxygrayanane diterpenoids, but also provided a basis to develop novel potent analgesics.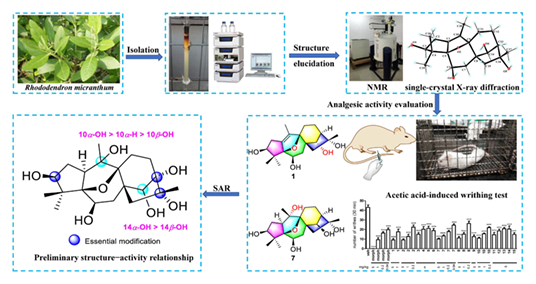
Supporting Information
| Filename | Description |
|---|---|
| cjoc202100077-sup-0001-Supinfo.pdfPDF document, 7.9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Li, Y.; Liu, Y. B.; Zhang, J. J.; Liu, Y.; Ma, S. G.; Qu, J.; Lv, H. N.; Yu, S. S. Antinociceptive grayanoids from the roots of Rhododendron molle. J. Nat. Prod. 2015, 78, 2887–2895.
- 2 Liu, C. C.; Lei, C.; Zhong, Y.; Gao, L. X.; Li, J. Y.; Yu, M. H.; Li, J.; Hou, A. J. Novel grayanane diterpenoids from Rhododendron principis. Tetrahedron 2014, 70, 4317–4322.
- 3 Li, Y.; Liu, Y. B.; Yan, H. M.; Liu, Y. L.; Li, Y. H.; Lv, H. N.; Ma, S. G.; Qu, J.; Yu, S. S. Rhodomollins A and B, two diterpenoids with an unprecedented backbone from the fruits of Rhododendron molle. Sci. Rep. 2016, 6, 36752.
- 4 Zhou, J. F.; Liu, T. T.; Zhang, H. Q; Zheng, G. J.; Qiu, Y.; Deng, M. Y.; Zhang, C.; Yao, G. M. Anti-inflammatory grayanane diterpenoids from the leaves of Rhododendron molle. J. Nat. Prod. 2018, 81, 151−161.
- 5 Li, C. H.; Zhang, J. Y.; Zhang, X. Y.; Li, S. H.; Gao, J. M. An overview of grayanane diterpenoids and their biological activities from the Ericaceae family in the last seven years. Eur. J. Med. Chem. 2019, 166, 400–416.
- 6 Li, Y.; Liu, Y. B.; Yu, S. S. Grayanoids from the Ericaceae family: structures, biological activities and mechanism of action. Phytochem. Rev. 2013, 12, 305–325.
- 7 Niu, C. S.; Li, Y.; Liu, Y. B.; Ma, S. G.; Wang, X. J.; Liu, F.; Liu, S.; Qu, J.; Yu, S. S. Diverse epoxy grayanane diterpenoids with analgesic activity from the roots of Pieris formosa. Fitoterapia 2019, 133, 29–34.
- 8 Niu, C. S.; Li, Y.; Liu, Y. B.; Ma, S. G.; Li, L.; Qu, J.; Yu, S. S. Analgesic diterpenoids from the twigs of Pieris formosa. Tetrahedron 2016, 72, 44–49.
- 9 Chen, S. N.; Bao, G. H.; Wang, L. Q.; Qin, G. W. Two new compounds from the flowers of Rhododendron molle. Chin. J. Nat. Med. 2013, 11, 525–527.
- 10 Zheng, G. J.; Zhou, J. F.; Huang, L.; Zhang, H.; Sun, N.; Zhang, H. Q.; Jin, P. F.; Yue, M. B.; Meng, L. K.; Yao, G. M. Antinociceptive grayanane diterpenoids from the leaves of Pieris japonica. J. Nat. Prod. 2019, 82, 3330−3339.
- 11 Zheng, G. J.; Jin, P. F.; Huang, L.; Sun, N.; Zhang, H. Q.; Meng, L. K.; Zhang, H.; Yue, M. B.; Yao, G. M. Grayanane diterpenoid glucosides as potent analgesics from Pieris japonica. Phytochemistry 2020, 171, 112234.
- 12 Sun, N.; Zheng, G. J.; He, M. J.; Feng, Y. Y.; Liu, J. J.; Wang, M. C.; Zhang, H. Q.; Zhou, J. F.; Yao, G. M. Grayanane diterpenoids from the leaves of Rhododendron auriculatum and their analgesic activities. J. Nat. Prod. 2019, 82, 1849–1860.
- 13 Li, Y.; Zhu, Y. X.; Zhang, Z. X.; Liu, Y. L.; Liu, Y. B.; Qu, J.; Ma, S. G.; Wang, X. J.; Yu, S. S. Diterpenoids from the fruits of Rhododendron molle, potent analgesics for acute pain. Tetrahedron 2018, 74, 693–699.
- 14 Li, Y.; Liu, Y. B.; Liu, Y. L.; Wang, C. ; Wu, L. Q.; Li, L.; Ma, S. G.; Qu, J.; Yu, S. S. Mollanol A, a diterpenoid with a new C-Nor-D-homograyanane skeleton from the fruits of Rhododendron molle. Org. Lett. 2014, 16, 4320–4323.
- 15 Zhu, Y. X.; Yan, H. M.; Wang, X. J.; Zhang, Z. X.; Zhang, H. P.; Chai, L. S.; Li, L.; Qu, J.; Li, Y. Micranthanosides I and II, two novel 1,10-secograyanane diterpenoids and their antinociceptive analogues from the leaves and twigs of Rhododendron micranthum. RSC Adv. 2019, 9, 18439–18450.
- 16 Li, C. H.; Luo, S. H.; Li, S. H.; Gao, J. M. New antifeedant grayanane diterpenoids from the flowers of Pieris formosa. Molecules 2017, 22, 1431/1–1431/9.
- 17 Hamanaka, N.; Miyakoshi, H.; Furusaki, A.; Matsumoto, T. Isolation and characterization of two new diterpenoids from Leucothoe grayana. Chem. Lett. 1972, 9, 779–782.
- 18 Sakakibara, J.; Shirai, N. Grayanoside D, a diterpene glucoside from Leucothoe grayana. Phytochemistry 1980, 19, 2159–2162.
- 19 Wang, L. Q.; Chen, S. N.; Qin, G. W.; Cheng, K. F. Grayanane diterpenoids from Pieris formosa. J. Nat. Prod. 1998, 61, 1473–1475.
- 20 Zhang, Z. R.; Zhong, J. D.; Li, H. M.; Li, H. Z.; Li, R. T.; Deng, X. L. Two new grayanane diterpenoids from the flowers of Rhododendron molle. J. Asian Nat. Prod. Res. 2012, 14, 764–768.
- 21 Chinese Materia Medica, Shanghai Scientific & Technical Publishers, Shanghai, 1999, Vol. 16, pp. 29–31.
- 22 Sun, N.; Zhu, Y.; Zhou, H. F.; Zhou, J. F.; Zhang, H. Q.; Zhang, M. K.; Zeng, H.; Yao, G. M. Grayanane Diterpenoid Glucosides from the Leaves of Rhododendron micranthum and their bioactivities evaluation. J. Nat. Prod. 2018, 81, 2673–2681.
- 23 Zhang, M. K.; Xie, Y. Y.; Zhan, G. Q.; Lei, L.; Shu, P. H.; Chen, Y. L.; Xue, Y. B.; Luo, Z. W.; Wan, Q.; Yao, G. M.; Zhang, Y. H. Grayanane and leucothane diterpenoids from the leaves of Rhododendron micranthum. Phytochemistry 2015, 117, 107–115.
- 24 Sun, N.; Qiu, Y.; Zhu, Y.; Liu, J. J.; Zhang, H. Q.; Zhang, Q. H.; Zhang, M. K.; Zheng, G. J.; Zhang, C.; Yao, G. M. Rhodomicranosides A-I, analgesic diterpene glucosides with diverse carbon skeletons from Rhododendron micranthum. Phytochemistry 2019, 158, 1–12.
- 25 Zhang, M. K.; Zhu, Y.; Zhan, G. Q.; Shu, P. H.; Sa, R. J.; Lei, L.; Xiang, M.; Xue, Y. B.; Luo, Z. W.; Wan, Q.; Yao, G. M.; Zhang, Y. H. Micranthanone A, a new diterpene with an unprecedented carbon skeleton from Rhododendron micranthum. Org. Lett. 2013, 15, 3094–3097.
- 26 Zheng, G. J.; Jin, P. F.; Huang, L.; Zhang, Q. H.; Meng, L. K.; Yao, G. M. Structurally diverse diterpenoids from Pieris japonica as potent analgesics. Bioorg. Chem. 2020, 99, 103794.
- 27 Sun, N.; Feng, Y. Y.; Zhang, Q. H.; Liu, J. J.; Zhou, H. F.; Zhang, H. Q.; Zheng, G. J.; Zhou, J. F.; Yao, G. M. Analgesic diterpenoids with diverse carbon skeletons from the leaves of Rhododendron auriculatum. Phytochemistry 2019, 168, 112113.
- 28 Zhou, J. F.; Sun, N.; Zhang, H. Q.; Zheng, G. J.; Liu, J. J.; Yao, G. M. Rhodomollacetals A-C, PTP1B inhibitory diterpenoids with a 2,3:5,6-di-seco-grayanane skeleton from the leaves of Rhododendron molle. Org. Lett. 2017, 19, 5352–5355.
- 29 Zhou, J. F.; Zhan, G. Q.; Zhang, H. Q.; Zhang, Q. H.; Li, Y.; Xue, Y. B.; Yao, G. M. Rhodomollanol A, a highly oxygenated diterpenoid with a 5/7/5/5 tetracyclic carbon skeleton from the leaves of Rhododendron molle. Org. Lett. 2017, 19, 3935–3938.
- 30 Zhou, J. F.; Liu, J. J.; Dang, T.; Zhou, H. F.; Zhang, H. Q.; Yao, G. M. Mollebenzylanols A and B, highly modified and functionalized diterpenoids with a 9-benzyl-8,10-dioxatricyclo[5.2.1.01,5]decane core from Rhododendron molle. Org. Lett. 2018, 20, 2063–2066.
- 31 Flack, H. D.; Bernardinelli, G. The use of X-ray crystallography to determine absolute configuration. Chirality 2008, 20, 681–690.
- 32 Teng, Y.; Zhang, H. Q.; Zhou, J. F.; Zhan, G. Q.; Yao, G. M. Hebecarposides A—K, antiproliferative lanostane-type triterpene glycosides from the leaves of Lyonia ovalifolia var. hebecarpa. Phytochemistry 2018, 151, 32–41.
- 33 Zheng, G. J.; Kadir, A.; Zheng, X. F.; Jin, P. F.; Liu, J. J.; Maiwulanjiang, M.; Yao, G. M.; Aisa, H. A. Spirodesertols A and B, two highly modified spirocyclic diterpenoids with an unprecedented 6-isopropyl-3H- spiro[benzofuran-2,1'-cyclohexane] motif from Salvia deserta. Org. Chem. Front. 2020, 7, 3137–3145.




