Rhodium(I)-Catalyzed Enantioselective C(sp3)—H Functionalization via Carbene-Induced Asymmetric Intermolecular C—H Insertion†
Bo Liu
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, 1088 Xueyuan Boulevard, Shenzhen, Guangdong, 518055 China
Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zuchongzhi Road, Shanghai, 201203 China
Search for more papers by this authorCorresponding Author
Ming-Hua Xu
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, 1088 Xueyuan Boulevard, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]Search for more papers by this authorBo Liu
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, 1088 Xueyuan Boulevard, Shenzhen, Guangdong, 518055 China
Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zuchongzhi Road, Shanghai, 201203 China
Search for more papers by this authorCorresponding Author
Ming-Hua Xu
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, 1088 Xueyuan Boulevard, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]Search for more papers by this author† Dedicated to Department of Chemistry, SUSTech, on the Occasion of Her 10th Anniversary.
Main observation and conclusion
Transition-metal-catalyzed C—H insertion of metal-carbene represents an excellent and powerful approach for C—H functionalization. However, despite remarkable advances in metal-carbene chemistry, transition metal catalysts that are capable of enantioselective intermolecular carbene C—H insertion are mainly constrained to dirhodium(II) and iridium(III)-based complexes. Herein, we disclose a new version of asymmetric carbene C—H insertion reaction with rhodium(I) catalyst. A highly enantioselective rhodium(I) complex-catalyzed C(sp3)—H functionalization of 1,4-cyclohexadienes with α-aryl-α-diazoacetates was successfully developed. By using chiral bicyclo[2.2.2]-octadiene as ligand, rhodium(I)-carbene-induced asymmetric intermolecular C—H insertion proceeds smoothly at room temperature, allowing access to a diverse variety of α-aryl-α-cyclohexadienyl acetates and gem-diaryl-containing acetates in good yields with good to excellent enantioselectivities (up to 99% ee). Furthermore, the synthetic utility of the reaction was highlighted by facile synthesis of a novel cannabinoid CB1 receptor ligand. This method may offer a new opportunity for the development of therapeutically exploitable cannabinoid receptor type ligands in medicinal chemistry. 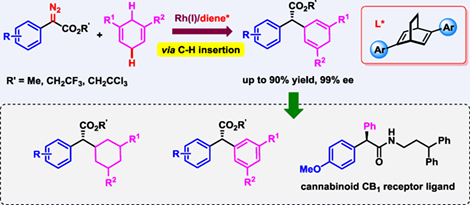
Supporting Information
| Filename | Description |
|---|---|
| cjoc202100040-sup-0001-Supinfo.pdfPDF document, 3.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Liu, Y.; You, T.; Wang, H.-X.; Tang, Z.; Zhou, C.-Y.; Che, C.-M. Iron- and cobalt-catalyzed C(sp3)–H bond functionalization reactions and their application in organic synthesis. Chem. Soc. Rev. 2020, 49, 5310–5358; (b) He, J.; Wasa, M.; Chan, K. S. L.; Shao, Q.; Yu, J.-Q. Palladium-Catalyzed Transformations of Alkyl C–H Bonds. Chem. Rev. 2017, 117, 8754–8786; (c) Ping, L.; Chung, D. S.; Bouffard, J.; Lee, S.-g. Transition metal-catalyzed site- and regio-divergent C–H bond functionalization. Chem. Soc. Rev. 2017, 46, 4299–4328.
- 2(a) Peña-López, M.; Beller, M. Functionalization of Unactivated C(sp3)−H Bonds Using Metal-Carbene Insertion Reactions. Angew. Chem. Int. Ed. 2017, 56, 46–48; (b) Caballero, A.; Díaz-Requejo, M. M.; Fructos, M. R.; Olmos, A.; Urbano, J.; Pérez, P. J. Catalytic functionalization of low reactive C(sp3)–H and C(sp2)–H bonds of alkanes and arenes by carbene transfer from diazo compounds. Dalton Trans. 2015, 44, 20295–20307; (c) Doyle, M. P.; Duffy, R.; Ratnikov, M.; Zhou, L. Catalytic Carbene Insertion into C−H Bonds. Chem. Rev. 2010, 110, 704–724; (d) Davies, H. M. L.; Manning, J. R. Catalytic C–H functionalization by metal carbenoid and nitrenoid insertion. Nature 2008, 451, 417–424; (e) Davies, H. M. L.; Beckwith, R. E. J. Catalytic Enantioselective C−H Activation by Means of Metal−Carbenoid-Induced C−H Insertion. Chem. Rev. 2003, 103, 2861–2904.
- 3(a) Dong, K.; Fan, X.; Pei, C.; Zheng, Y.; Chang, S.; Cai, J.; Qiu, L.; Yu, Z.-X.; Xu, X. Transient-axial-chirality controlled asymmetric rhodium-carbene C(sp2)-H functionalization for the synthesis of chiral fluorenes. Nat. Commun. 2020, 11, 2363; (b) Yu, Z.; Li, Y.; Zhang, P.; Liu, L.; Zhang, J. Ligand and counteranion enabled regiodivergent C–H bond functionalization of naphthols with α-aryl-α-diazoesters. Chem. Sci. 2019, 10, 6553–6559; (c) Xu, B.; Li, M.-L.; Zuo, X.-D.; Zhu, S.-F.; Zhou, Q.-L. Catalytic Asymmetric Arylation of α-Aryl-α-diazoacetates with Aniline Derivatives. J. Am. Chem. Soc. 2015, 137, 8700–8703; (d) Yu, Z.; Ma, B.; Chen, M.; Wu, H.-H.; Liu, L.; Zhang, J. Highly Site-Selective Direct C–H Bond Functionalization of Phenols with α-Aryl-α-diazoacetates and Diazooxindoles via Gold Catalysis. J. Am. Chem. Soc. 2014, 136, 6904–6907; (e) Liao, K.; Yang, Y.-F.; Li, Y.; Sanders, J. N.; Houk, K. N.; Musaev, D. G.; Davies, H. M. L. Design of catalysts for site-selective and enantioselective functionalization of non-activated primary C–H bonds. Nat. Chem. 2018, 10, 1048–1055; (f) Fu, J.; Ren, Z.; Bacsa, J.; Musaev, D. G.; Davies, H. M. L. Desymmetrization of cyclohexanes by site- and stereoselective C–H functionalization. Nature 2018, 564, 395–399; (g) Liao, K.; Pickel, T. C.; Boyarskikh, V.; Bacsa, J.; Musaev, D. G.; Davies, H. M. L. Site-selective and stereoselective functionalization of non-activated tertiary C–H bonds. Nature 2017, 551, 609–613; (h) Liao, K.; Negretti, S.; Musaev, D. G.; Bacsa, J.; Davies, H. M. L. Site-selective and stereoselective functionalization of unactivated C–H bonds. Nature 2016, 533, 230–234.
- 4(a) Liao, K.; Liu, W.; Niemeyer, Z. L.; Ren, Z.; Bacsa, J.; Musaev, D. G.; Sigman, M. S.; Davies, H. M. L. Site-Selective Carbene-Induced C–H Functionalization Catalyzed by Dirhodium Tetrakis(triarylcyclopropanecarboxylate) Complexes. ACS Catal. 2018, 8, 678–682; (b) Bedell, T. A.; Hone, G. A. B.; Valette, D.; Yu, J.-Q.; Davies, H. M. L.; Sorensen, E. J. Rapid Construction of a Benzo-Fused Indoxamycin Core Enabled by Site-Selective C−H Functionalizations. Angew. Chem. Int. Ed. 2016, 55, 8270–8274.
- 5(a) Suematsu, H.; Katsuki, T. Iridium(III) Catalyzed Diastereo- and Enantioselective C−H Bond Functionalization. J. Am. Chem. Soc. 2009, 131, 14218–14219; (b) Wang, J.-C.; Xu, Z.-J.; Guo, Z.; Deng, Q.-H.; Zhou, C.-Y.; Wan, X.-L.; Che, C.-M. Highly enantioselective intermolecular carbene insertion to C–H and Si–H bonds catalyzed by a chiral iridium(iii) complex of a D4-symmetric Halterman porphyrin ligand. Chem. Commun. 2012, 48, 4299–4301; (c) Owens, C. P.; Varela-Álvarez, A.; Boyarskikh, V.; Musaev, D. G.; Davies, H. M. L.; Blakey, S. B. Iridium(iii)-bis(oxazolinyl)phenyl catalysts for enantioselective C–H functionalization. Chem. Sci. 2013, 4, 2590–2596.
- 6(a) Davies, H. M. L.; Stafford, D. G.; Hansen, T. Catalytic Asymmetric Synthesis of Diarylacetates and 4,4-Diarylbutanoates. A Formal Asymmetric Synthesis of (+)-Sertraline. Org. Lett. 1999, 1, 233–236; (b) Denton, J. R.; Davies, H. M. L. Enantioselective Reactions of Donor/Acceptor Carbenoids Derived from α-Aryl-α-Diazoketones. Org. Lett. 2009, 11, 787–790; (c) Müller, P.; Tohill, S. Intermolecular Cyclopropanation versus CH Insertion in RhII-Catalyzed Carbenoid Reactions. Tetrahedron 2000, 56, 1725–1731.
- 7(a) Li, Y.; Xu, M.-H. Simple sulfur–olefins as new promising chiral ligands for asymmetric catalysis. Chem. Commun. 2014, 50, 3771–3782; (b) Wang, Z.-Q.; Feng, C.-G.; Xu, M.-H.; Lin, G.-Q. Design of C2-Symmetric Tetrahydropentalenes as New Chiral Diene Ligands for Highly Enantioselective Rh-Catalyzed Arylation of N-Tosylarylimines with Arylboronic Acids. J. Am. Chem. Soc. 2007, 129, 5336–5337; (c) Zhu, T.-S.; Jin, S.-S.; Xu, M.-H. Rhodium-Catalyzed, Highly Enantioselective 1,2-Addition of Aryl Boronic Acids to α-Ketoesters and α-Diketones Using Simple, Chiral Sulfur–Olefin Ligands. Angew. Chem. Int. Ed. 2012, 51, 780–783; (d) Wang, H.; Jiang, T.; Xu, M.-H. Simple Branched Sulfur–Olefins as Chiral Ligands for Rh-Catalyzed Asymmetric Arylation of Cyclic Ketimines: Highly Enantioselective Construction of Tetrasubstituted Carbon Stereocenters. J. Am. Chem. Soc. 2013, 135, 971–974.
- 8(a) Chen, D.; Zhang, X.; Qi, W.-Y.; Xu, B.; Xu, M.-H. Rhodium(I)-Catalyzed Asymmetric Carbene Insertion into B–H Bonds: Highly Enantioselective Access to Functionalized Organoboranes. J. Am. Chem. Soc. 2015, 137, 5268–5271; (b) Chen, D.; Zhu, D.-X.; Xu, M.-H. Rhodium(I)- Catalyzed Highly Enantioselective Insertion of Carbenoid into Si–H: Efficient Access to Functional Chiral Silanes. J. Am. Chem. Soc. 2016, 138, 1498–1501.
- 9(a) Nishimura, T.; Maeda, Y.; Hayashi, T. Asymmetric Cyclopropanation of Alkenes with Dimethyl Diazomalonate Catalyzed by Chiral Diene–Rhodium Complexes. Angew. Chem. Int. Ed. 2010, 49, 7324–7327; (b) Guo, R.-T.; Zhang, Y.-L.; Tian, J.-J.; Zhu, K.-Y.; Wang, X.-C. Rhodium-Catalyzed ortho-Selective Carbene C–H Insertion of Unprotected Phenols Directed by a Transient Oxonium Ylide Intermediate. Org. Lett. 2020, 22, 908–913; (c) Yada, A.; Fujita, S.; Murakami, M. Enantioselective Insertion of a Carbenoid Carbon into a C–C Bond to Expand Cyclobutanols to Cyclopentanols. J. Am. Chem. Soc. 2014, 136, 7217–7220; (d) Ma, X.; Jiang, J.; Lv, S.; Yao, W.; Yang, Y.; Liu, S.; Xia, F.; Hu, W. An Ylide Transformation of Rhodium(I) Carbene: Enantioselective Three-Component Reaction through Trapping of Rhodium(I)-Associated Ammonium Ylides by β-Nitroacrylates. Angew. Chem. Int. Ed. 2014, 53, 13136–13139.
- 10 Davies, H. M. L.; Gregg, T. M. Asymmetric synthesis of (+)-indatraline using rhodium-catalyzed C–H activation. Tetrahedron Lett. 2002, 43, 4951–4953.
- 11TLC indicates the formation of approximately 1 : 1 diastereomers.
- 12(a) Urbani, P.; Cascio, M. G.; Ramunno, A.; Bisogno, T.; Saturnino, C.; Marzo, V. D. Novel sterically hindered cannabinoid CB1 receptor ligands. Bioorg. Med. Chem. 2008, 16, 7510–7515; (b) De Freitas, G. B. L.; da Silva, L. L.; Romeiro, N. C.; Fraga, C. A. M. Development of CoMFA and CoMSIA models of affinity and selectivity for indole ligands of cannabinoid CB1 and CB2 receptors. Eur. J. Med. Chem. 2009, 44, 2482–2496; (c) Brogi, S.; Corelli, F.; Di Marzo, V.; Ligresti, A.; Mugnaini, C.; Pasquini, S.; Tafi, A. Three-dimensional quantitative structure-selectivity relationships analysis guided rational design of a highly selective ligand for the cannabinoid receptor 2. Eur. J. Med. Chem. 2011, 46, 547–555.




