Synthesis of 3-Methylthioindoles via Intramolecular Cyclization of 2-Alkynylanilines Mediated by DMSO/DMSO-d6 and SOCl2
Xuemin Li
Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China, State Key Laboratory of Microbial Technology, Institute of Microbial Technology, Shandong University, Qingdao, Shandong, 266237 China
Search for more papers by this authorBeibei Zhang
Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China, State Key Laboratory of Microbial Technology, Institute of Microbial Technology, Shandong University, Qingdao, Shandong, 266237 China
Search for more papers by this authorJingran Zhang
Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China, State Key Laboratory of Microbial Technology, Institute of Microbial Technology, Shandong University, Qingdao, Shandong, 266237 China
Search for more papers by this authorXi Wang
Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China, State Key Laboratory of Microbial Technology, Institute of Microbial Technology, Shandong University, Qingdao, Shandong, 266237 China
Search for more papers by this authorDongke Zhang
Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China, State Key Laboratory of Microbial Technology, Institute of Microbial Technology, Shandong University, Qingdao, Shandong, 266237 China
Search for more papers by this authorCorresponding Author
Yunfei Du
Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China, State Key Laboratory of Microbial Technology, Institute of Microbial Technology, Shandong University, Qingdao, Shandong, 266237 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Kang Zhao
Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China, State Key Laboratory of Microbial Technology, Institute of Microbial Technology, Shandong University, Qingdao, Shandong, 266237 China
E-mail: [email protected]; [email protected]Search for more papers by this authorXuemin Li
Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China, State Key Laboratory of Microbial Technology, Institute of Microbial Technology, Shandong University, Qingdao, Shandong, 266237 China
Search for more papers by this authorBeibei Zhang
Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China, State Key Laboratory of Microbial Technology, Institute of Microbial Technology, Shandong University, Qingdao, Shandong, 266237 China
Search for more papers by this authorJingran Zhang
Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China, State Key Laboratory of Microbial Technology, Institute of Microbial Technology, Shandong University, Qingdao, Shandong, 266237 China
Search for more papers by this authorXi Wang
Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China, State Key Laboratory of Microbial Technology, Institute of Microbial Technology, Shandong University, Qingdao, Shandong, 266237 China
Search for more papers by this authorDongke Zhang
Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China, State Key Laboratory of Microbial Technology, Institute of Microbial Technology, Shandong University, Qingdao, Shandong, 266237 China
Search for more papers by this authorCorresponding Author
Yunfei Du
Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China, State Key Laboratory of Microbial Technology, Institute of Microbial Technology, Shandong University, Qingdao, Shandong, 266237 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Kang Zhao
Tianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China, State Key Laboratory of Microbial Technology, Institute of Microbial Technology, Shandong University, Qingdao, Shandong, 266237 China
E-mail: [email protected]; [email protected]Search for more papers by this authorMain observation and conclusion
The intramolecular cyclization of 2-alkynylanilines mediated by DMSO/SOCl2 was found to afford biologically interesting 3-methylthioindoles, which are rarely obtained by the exiting methods. DMSO could also be replaced with its deuterated counterpart, enabling the method applicable to the construction of indole skeleton bearing a SCD3 moiety at its 3-position.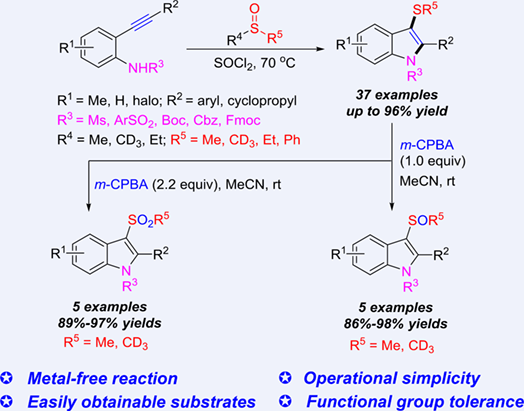
Supporting Information
| Filename | Description |
|---|---|
| cjoc202000701-sup-0001-Supinfo.pdfPDF document, 7.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Kawasaki, T.; Higuchi, K. Simple Indole Alkaloids and Those with a Nonrearranged Monoterpenoid Unit. Nat. Prod. Rep. 2005, 22, 761–793; (b) Vicente, R. Recent Advances in Indole Syntheses: New Routes for a Classic Target. Org. Biomol. Chem. 2011, 9, 6469–6480; (c) Inman, M.; Moody, C. J. Indole Synthesis – Something Old, Something New. Chem. Sci. 2013, 4, 29–41; (d) Ishikura, M.; Abe, T.; Choshi, T.; Hibino, S. Simple Indole Alkaloids and Those with a Nonrearranged Monoterpenoid Unit. Nat. Prod. Rep. 2015, 32, 1389–1471; (e) Park, J.; Kim, D.-H.; Das, T.; Cho, C.-G. Intramolecular Fischer Indole Synthesis for the Direct Synthesis of 3,4-Fused Tricyclic Indole and Application to the Total Synthesis of (−)-Aurantioclavine. Org. Lett. 2016, 18, 5098–5101; (f) Ning, X.-S.; Liang, X.; Hu, K.-F.; Yao, C.-Z.; Qu, J.-P.; Kang, Y.-B. Pd-tBuONO Cocatalyzed Aerobic Indole Synthesis. Adv. Synth. Catal. 2018, 360, 1590–1594; (g) Mao, J.; Wang, Z.; Xu, X.; Liu, G.; Jiang, R.; Guan, H.; Zheng, Z.; Walsh, P. J. Synthesis of Indoles through Domino Reactions of 2-Fluorotoluenes and Nitriles. Angew. Chem. Int. Ed. 2019, 58, 11033–11038.
- 2(a) Buechi, G.; Gould, S. J.; Naef, F. Stereospecific Syntheses of Uleine and Epiuleine. J. Am. Chem. Soc. 1971, 93, 2492–2501; (b) Garbe, T. R.; Kobayashi, M.; Shimizu, N.; Takesue, N.; Ozawa, M.; Yukawa, H. Indolyl Carboxylic Acids by Condensation of Indoles with α-Keto Acids. J. Nat. Prod. 2000, 63, 596–598; (c) Alparslan, A.; Ulf, P. Chemistry and Biology of New Marine Alkaloids from the Indole and Annelated Indole Series. Curr. Med. Chem. 2003, 10, 1113–1127; (d) Feng, T.; Cai, X.-H.; Liu, Y.-P.; Li, Y.; Wang, Y.-Y.; Luo, X.-D. Melodinines A−G, Monoterpenoid Indole Alkaloids from Melodinus Henryi. J. Nat. Prod. 2010, 73, 22–26; (e) Kochanowska-Karamyan, A. J.; Hamann, M. T. Marine Indole Alkaloids: Potential New Drug Leads for the Control of Depression and Anxiety. Chem. Rev. 2010, 110, 4489–4497; (f) Mizoguchi, H.; Oikawa, H.; Oguri, H. Biogenetically Inspired Synthesis and Skeletal Diversification of Indole Alkaloids. Nat. Chem. 2014, 6, 57–64.
- 3(a) Leena, G.; Archna, T.; Prem, M. S. C. Bis and Tris Indole Alkaloids from Marine Organisms: New Leads for Drug Discovery. Curr. Med. Chem. 2007, 14, 1789–1803; (b) Humphrey, G. R.; Kuethe, J. T. Practical Methodologies for the Synthesis of Indoles. Chem. Rev. 2006, 106, 2875–2911; (c) Horton, D. A.; Bourne, G. T.; Smythe, M. L. The Combinatorial Synthesis of Bicyclic Privileged Structures or Privileged Substructures. Chem. Rev. 2003, 103, 893–930; (d) Evans, B. E.; Rittle, K. E.; Bock, M. G.; Dipardo, R. M.; Freidinger, R. M.; Whitter, W. L.; Lundell, G. F.; Veber, D. F.; Anderson, P. S.; Chang, R. S. L.; Lotti, V. J.; Cerino, D. J.; Chen, T. B.; Kling, P. J.; Kunkel, K. A.; Springer, J. P.; Hirshfield, J. Methods for Drug Discovery: Development of Potent, Selective, Orally Effective Cholecystokinin Antagonists. J. Med. Chem. 1988, 31, 2235–2246; (e) Rieck, G. C.; Fiander, A. N. Human Papillomavirus, Cervical Carcinogenesis and Chemoprevention with Indole Derivates – A Review of Pathomechanisms. Mol. Nutr. Food Res. 2008, 52, 105–113.
- 4(a) De Martino, G.; La Regina, G.; Coluccia, A.; Edler, M. C.; Barbera, M. C.; Brancale, A.; Wilcox, E.; Hamel, E.; Artico, M.; Silvestri, R. Arylthioindoles, Potent Inhibitors of Tubulin Polymerization. J. Med. Chem. 2004, 47, 6120–6123; (b) Zhang, M.-Z.; Chen, Q.; Yang, G.-F. A Review on Recent Developments of Indole-containing Antiviral Agents. Eur. J. Med. Chem. 2015, 89, 421–441; (c) Cianchi, F.; Cortesini, C.; Magnelli, L.; Fanti, E.; Papucci, L.; Schiavone, N.; Messerini, L.; Vannacci, A.; Capaccioli, S.; Perna, F.; Lulli, M.; Fabbroni, V.; Perigli, G.; Bechi, P.; Masini, E. Inhibition of 5-Lipoxygenase by MK886 Augments the Antitumor Activity of Celecoxib in Human Colon Cancer Cells. Mol. Cancer Ther. 2006, 5, 2716–2726.
- 5(a) Grime, K.; Pehrson, R.; Nordell, P.; Gillen, M.; Kühn, W.; Mant, T.; Brännström, M.; Svanberg, P.; Jones, B.; Brealey, C. An S-warfarin and AZD1981 Interaction: In Vitro and Clinical Pilot Data Suggest the N-deacetylated Amino Acid Metabolite as the Primary Perpetrator. Br. J. Clin. Pharmacol. 2017, 83, 381–392; (b) Schmidt, J.; Bell, F.; Akam, E.; Marshall, C.; Dainty, I.; Heinemann, A.; Dougall, I.; Bonnert, R.; Sargent, C. Biochemical and Pharmacological Characterization of AZD1981, an Orally Available Selective DP2 Antagonist in Clinical Development for Asthma. Br. J. Pharmacol. 2013, 168, 1626–1638.
- 6(a) Johal, K. J.; Saini, S. S. Current and Emerging Treatments for Chronic Spontaneous Urticaria. Ann. Allergy. Asthma. Immunol. 2020, 125, 380–387.
- 7(a) Funk, C. D. Leukotriene Modifiers as Potential Therapeutics for Cardiovascular Disease. Nat. Rev. Drug Discovery 2005, 4, 664–672; (b) Hutchinson, J. H.; Charleson, S.; Evans, J. F.; Falgueyret, J.-P.; Hoogsteen, K.; Jones, T. R.; Kargman, S.; Macdonald, D.; Mcfarlane, C. S. Thiopyranol[2,3,4-c,d]indoles as Inhibitors of 5-Lipoxygenase, 5-Lipoxygenase-activating Protein, and Leukotriene C4 Synthase. J. Med. Chem. 1995, 38, 4538–4547; (c) Chang, L. C.; Wang, J. P. The Upstream Regulation of p38 Mitogen-activated Protein Kinase Phosphorylation by Arachidonic Acid in Rat Neutrophils. J. Pharm. Pharmacol. 2000, 52, 539–546.
- 8 De Martino, G.; Edler, M. C.; La Regina, G.; Coluccia, A.; Barbera, M. C.; Barrow, D.; Nicholson, R. I.; Chiosis, G.; Brancale, A.; Hamel, E.; Artico, M.; Silvestri, R. New Arylthioindoles: Potent Inhibitors of Tubulin Polymerization. 2. Structure-Activity Relationships and Molecular Modeling Studies. J. Med. Chem. 2006, 49, 947–954.
- 9(a) Yang, X.; Bao, Y.; Dai, Z.; Zhou, Q.; Yang, F. Catalyst-free Sulfenylation of Indoles with Sulfinic Esters in Ethanol. Green Chem. 2018, 20, 3727–3731; (b) Huang, X.; Chen, Y.; Zhen, S.; Song, L.; Gao, M.; Zhang, P.; Li, H.; Yuan, B.; Yang, G. Cobalt-Catalyzed Aerobic Cross-dehydro- genative Coupling of C–H and Thiols in Water for C–S Formation. J. Org. Chem. 2018, 83, 7331–7340; (c) Sang, P.; Chen, Z.; Zou, J.; Zhang, Y. K2CO3 Promoted Direct Sulfenylation of Indoles: A Facile Approach Towards 3-Sulfenylindoles. Green Chem. 2013, 15, 2096–2100; (d) Li, W.; Wang, H.; Liu, S.; Feng, H.; Benassi, E.; Qian, B. Iodine/Manganese Catalyzed Sulfenylation of Indole via Dehydrogenative Oxidative Coupling in Anisole. Adv. Synth. Catal. 2020, 362, 2666–2671.
- 10 He, X.-L.; Majumder, S.; Wu, J.; Jin, C.-D.; Guo, S.-R.; Guo, Z.-P.; Yang, M. Metal- and Phosphine-free Electrophilic Vicinal Chloro-alkylthiolation and Trifluoromethylthiolation of Indoles Using Sodium Sulfinate in the Presence of Triphosgene. Org. Chem. Front. 2019, 6, 2435–2440.
- 11 Chen, Y.; Cho, C.-H.; Larock, R. C. A Novel Synthetic Route to 3-Sulfenyl- and 3-Selenylindoles by n-Bu4NI-induced Electrophilic Cyclization. Org. Lett. 2009, 11, 173–176.
- 12(a) Du, H.-A.; Tang, R.-Y.; Deng, C.-L.; Liu, Y.; Li, J.-H.; Zhang, X.-G. Iron-Facilitated Iodine-mediated Electrophilic Annulation of N,N-dimethyl-2-alkynylanilines with Disulfides or Diselenides. Adv. Synth. Catal. 2011, 353, 2739–2748; (b) Shi, Q.; Li, P.; Zhang, Y.; Wang, L. Visible Light-induced Tandem Oxidative Cyclization of 2-Alkynylanilines with Disulfides (Diselenides) to 3-Sulfenyl- and 3-Selenylindoles under Transition Metal-free and Photocatalyst-free Conditions. Org. Chem. Front. 2017, 4, 1322–1330; (c) Guo, Y.-J.; Tang, R.-Y.; Li, J.-H.; Zhong, P.; Zhang, X.-G. Palladium-catalyzed Annulation of 2-(1-Alkynyl)benzenamines with Disulfides: Synthesis of 3-Sulfenylindoles. Adv. Synth. Catal. 2009, 351, 2615–2618.
- 13(a) Meesin, J.; Pohmakotr, M.; Reutrakul, V.; Soorukram, D.; Leowanawat, P.; Kuhakarn, C. Synthesis of N-alkyl-3-sulfonylindoles and N-alkyl-3-sulfanylindoles by Cascade Annulation of 2-Alkynyl-N, N-dialkylanilines. Org. Biomol. Chem. 2017, 15, 3662–3669; (b) Sharma, S.; Pathare, R. S.; Sukanya; Maurya, A. K.; Goswami, B.; Agnihotri, V. K.; Sawant, D. M.; Pardasani, R. T. Microwave Assisted Metal-/Oxidant-free Cascade Electrophilic Sulfenylation/5-endo-dig Cyclization of 2-Alkynylanilines to Generate Diversified 3-Sulfenylindoles. Tetrahedron Lett. 2017, 58, 3823–3826.
- 14 Han, D.; Li, Z.; Fan, R. Oxidative Nucleophilic Cyclization of 2-Alkynylanilines with Thiophenols under Metal-free Conditions. Org. Lett. 2014, 16, 6508–6511.
- 15(a) Hamel, P.; Zajac, N.; Atkinson, J. G.; Girard, Y. Nonreductive Desulfenylation of 3-Indolyl Sulfides. Improved Syntheses of 2-Substituted Indoles and 2-Indolyl Sulfides. J. Org. Chem. 1994, 59, 6372–6377; (b) Pfaffenbach, M.; Gaich, T. A Flexible Route to Indole Scaffolds – Formal Synthesis of (±)-Mersicarpine. Eur. J. Org. Chem. 2015, 2015, 3427–3429.
- 16(a) Gassman, P. G.; Van Bergen, T. J. Simple Method for the Conversion of Anilines into 2-Substituted Indoles. J. Am. Chem. Soc. 1973, 95, 590–591; (b) Gassman, P. G.; Van Bergen, T. J. Use of Methylthioacetaldehyde in the Synthesis of Indole and Its Derivatives. J. Am. Chem. Soc. 1973, 95, 591–592.
- 17(a) Meng, Y.; Wang, M.; Jiang, X. Multicomponent Reductive Cross- coupling of an Inorganic Sulfur Dioxide Surrogate: Straightforward Construction of Diversely Unctionalized Sulfones. Angew. Chem. Int. Ed. 2020, 59, 1346–1353; (b) Chen, J.; Chang, D.; Xiao, F.; Deng, G.-J. Four-component Quinazoline Synthesis from Simple Anilines, Aromatic Aldehydes and Ammonium Iodide under Metal-free Conditions. Green Chem. 2018, 20, 5459–5463; (c) Wu, X.; Gao, Q.; Geng, X.; Zhang, J.; Wu, Y.-D.; Wu, A.-X. Iodine-Promoted Oxidative Cross- coupling of Unprotected Anilines with Methyl Ketones: a Site-selective Direct C–H Bond Functionalization to C4-Dicarbonyl- ation of Anilines. Org. Lett. 2016, 18, 2507–2510; (d) Song, S.; Li, X.; Sun, X.; Yuan, Y.; Jiao, N. Efficient Bromination of Olefins, Alkynes, and Ketones with Dimethyl Sulfoxide and Hydrobromic Acid. Green Chem. 2015, 17, 3285–3289; (e) Liang, Y.-F.; Li, X.; Wang, X.; Zou, M.; Tang, C.; Liang, Y.; Song, S.; Jiao, N. Conversion of Simple Cyclohexanones into Catechols. J. Am. Chem. Soc. 2016, 138, 12271–12277; (f) Wu, Y.; Huang, Z.; Luo, Y.; Liu, D.; Deng, Y.; Yi, H.; Lee, J.-F.; Pao, C.-W.; Chen, J.-L.; Lei, A. X-ray Absorption and Electron Paramagnetic Resonance Guided Discovery of the Cu-catalyzed Synthesis of Multiaryl-substituted Furans from Aryl Styrene and Ketones Using DMSO as the Oxidant. Org. Lett. 2017, 19, 2330–2333; (g) Smith, L. H. S.; Coote, S. C.; Sneddon, H. F.; Procter, D. J. Beyond the Pummerer Reaction: Recent Developments in Thionium Ion Chemistry. Angew. Chem. Int. Ed. 2010, 49, 5832–5844; (h) Shen, W.-G.; Wu, Q.-Y.; Gong, X.-Y.; Ao, G.-Z.; Liu, F. A Facile Method for Hydroxytrifluoromethylation of Alkenes with Langlois Reagent and DMSO. Green Chem. 2019, 21, 2983–2987; (i) Mancuso, A. J.; Brownfain, D. S.; Swern, D. Structure of the Dimethyl Sulfoxide-oxalyl Chloride Reaction Product. Oxidation of Heteroaromatic and Diverse Alcohols to Carbonyl Compounds. J. Org. Chem. 1979, 44, 4148–4150.
- 18(a) Liu, F.-L.; Chen, J.-R.; Zou, Y.-Q.; Wei, Q.; Xiao, W.-J. Three-Component Coupling Reaction Triggered by Insertion of Arynes into the S=O Bond of DMSO. Org. Lett. 2014, 16, 3768–3771; (b) Chu, L.; Yue, X.; Qing, F.-L. Cu(II)-Mediated Methylthiolation of Aryl C−H Bonds with DMSO. Org. Lett. 2010, 12, 1644–1647; (c) Luo, F.; Pan, C.; Li, L.; Chen, F.; Cheng, J. Copper-mediated Methylthiolation of Aryl Halides with DMSO. Chem. Commun. 2011, 47, 5304–5306; (d) Gao, X.; Pan, X.; Gao, J.; Jiang, H.; Yuan, G.; Li, Y. NH4I-Mediated Three-component Coupling Reaction: Metal-free Synthesis of β-Alkoxy Methyl Sulfides from DMSO, Alcohols, and Styrenes. Org. Lett. 2015, 17, 1038–1041; (e) Wang, M.; Xiang, J.-C.; Cheng, Y.; Wu, Y.-D.; Wu, A.-X. Synthesis of 2,4,5-Trisubstituted Furans via a Triple C(sp3)–H Functionalization Reaction Using Rongalite as the C1 Unit. Org. Lett. 2016, 18, 524–527; (f) Hazarika, H.; Neog, K.; Sharma, A.; Das, B.; Gogoi, P. Three-Component Coupling Reactions of Aryne, DMSO, and Activated Alkyne: Stereoselective Synthesis of 2-[(o-methylthio)aryloxy]-substituted Dialkyl Maleates. J. Org. Chem. 2019, 84, 5846–5854; (g) Rather, S. A.; Kumar, A.; Ahmed, Q. N. Iodine–DMSO-promoted Divergent Reactivities of Arylacetylenes. Chem. Commun. 2019, 55, 4511–4514; (h) An, X.; Zhang, B.; Li, X.; Du, T.; Ai, Z.; Zhang, C.; Xu, J.; Sun, F.; Zhang, Y.; Du, Y. Construction of 4-(Methylthio)isochrome- nones Skeleton through Regioselective Intramolecular Cyclization of 2-Alkynylbenzoate Mediated by DMSO/[D6]DMSO and SOCl2. Eur. J. Org. Chem. 2020, 2020, 852–859; (i) Bates, D. K.; Sell, B. A.; Picard, J. A. An Interrupted Pummerer Reaction Induced by Vilsmeier Reagent (POCl3/DMF). Tetrahedron Lett. 1987, 28, 3535–3538.
- 19For a previous report describing the methylthiolation of indole skeleton via DMSO and (COCl)2, see: Zou, J.-F.; Huang, W.-S.; Li, L.; Xu, Z.; Zheng, Z.-J.; Yang, K.-F.; Xu, L.-W. DMSO as Oxidant and Sulfenylating Agent for Metal-free Oxidation and Methylthiolation of Alcohol- containing Indoles. RSC Adv. 2015, 5, 30389–30393.
- 20 Zhang, B.-B.; Li, X.-X.; Li, X.-M.; Sun, F.-X.; Du, Y.-F. Synthesis of 3-Methylthio-benzo[b]furans/thiophenes via Intramolecular Cyclization of 2-Alkynylanisoles/Sulfides Mediated by DMSO/DMSO-d6 and SOCl2. Chin. J. Chem. 2020, 37, 887–895.
- 21CCDC-2042618 (compound 2a) contains the supplementary crystallographic data for this work. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam. ac.uk/data_request/cif.
- 22Reducing the temperature did not lead to a better result.
- 23(a) Joseph, P. J. A.; Priyadarshini, S.; Kantam, M. L.; Sreedhar, B. Investigation of the Scope and Mechanism of Copper Catalyzed Regioselective Methylthiolation of Aryl Halides. Tetrahedron 2013, 69, 8276–8283; (b) Jones-Mensah, E.; Magolan, J. Aryl Methyl Sulfides via SNAr Using DMSO as the Source of the Thiomethyl Moiety. Tetrahedron Lett. 2014, 55, 5323–5326; (c) Ghosh, K.; Ranjit, S.; Mal, D. A Convenient Method for the Synthesis of Aryl Methyl Sulfides via Cu(I)-Mediated Methylthiolation of Haloarenes with DMSO. Tetrahedron Lett. 2015, 56, 5199–5202.
- 24(a) Omura, K.; Swern, D. Oxidation of Alcohols by “Activated” Dimethyl Sulfoxide. a Preparative, Steric and Mechanistic Study. Tetrahedron 1978, 34, 1651–1660; (b) Bellesia, F.; Boni, M.; Ghelfi, F.; Pagnoni, U. M.; Pinetti, A. β-Chloroalkyl Sulfides from Me2S/SO2Cl2/ Me2SO and Alkenes. Synth. Commun. 1992, 22, 1101–1108; (c) Zhang, T.; Dai, Y.; Cheng, S.; Liu, Y.; Yang, S.; Sun, B.; Tian, H. A Facile Method for the Sulfenyllactonization of Alkenoic Acids using Dimethyl Sulfoxide Activated by Oxalyl Chloride. Synthesis 2017, 49, 1380–1386.
- 25 Lucchini, V.; Modena, G.; Valle, G.; Capozzi, G. Stability and Reactivity of Thiirenium Ions. Dependence on Alkyl or Aryl Substitution at Ring Carbons. J. Org. Chem. 1981, 46, 4720–4724.
- 26(a) Wang, N.; Saidhareddy, P.; Jiang, X. Construction of Sulfur-containing Moieties in the Total Synthesis of Natural Products. Nat. Prod. Rep. 2020, 37, 246–275; (b) Kalgutkar, A. S.; Kozak, K. R.; Crews, B. C.; Hochgesang, G. P.; Marnett, L. J. Covalent Modification of Cyclooxygenase-2 (COX-2) by 2-Acetoxyphenyl Alkyl Sulfides, A New Class of Selective COX-2 Inactivators. J. Med. Chem. 1998, 41, 4800–4818; (c) Chang, M.-Y.; Chen, H.-Y.; Tsai, Y.-L. NH2OH–HCl-Mediated Umpolung α-Methylsulfonylation of α-Sulfonyl Ketones with Methylsulfoxides: Synthesis of α,β-Bis-sulfonyl Arylketones. Org. Lett. 2019, 21, 1832–1836; (d) Lu, M.; Qin, H.; Lin, Z.; Huang, M.; Weng, W.; Cai, S. Visible-Light-Enabled Oxidative Alkylation of Unactivated Alkenes with Dimethyl Sulfoxide through Concomitant 1,2-Aryl Migration. Org. Lett. 2018, 20, 7611–7615.
- 27(a) Bernotas, R. C.; Antane, S.; Shenoy, R.; Le, V.-D.; Chen, P.; Harrison, B. L.; Robichaud, A. J.; Zhang, G. M.; Smith, D.; Schechter, L. E. 3-(Arylsulfonyl)-1-(azacyclyl)-1H-indoles are 5-HT6 Receptor Modulators. Bioorg. Med. Chem. Lett. 2010, 20, 1657–1660; (b) Silvestri, R.; De Martino, G.; La Regina, G.; Artico, M.; Massa, S.; Vargiu, L.; Mura, M.; Loi, A. G.; Marceddu, T.; La Colla, P. Novel Indolyl Aryl Sulfones Active Against HIV-1 Carrying NNRTI Resistance Mutations: Synthesis and SAR Studies. J. Med. Chem. 2003, 46, 2482–2493; (c) Ragno, R.; Coluccia, A.; La Regina, G.; De Martino, G.; Piscitelli, F.; Lavecchia, A.; Novellino, E.; Bergamini, A.; Ciaprini, C.; Sinistro, A.; Maga, G.; Crespan, E.; Artico, M.; Silvestri, R. Design, Molecular Modeling, Synthesis, and Anti-HIV-1 Activity of New Indolyl Aryl Sulfones. Novel Derivatives of the Indole-2-carboxamide. J. Med. Chem. 2006, 49, 3172–3184.
- 28 Rajeshkumar, V.; Neelamegam, C.; Anandan, S. A One-pot Metal-free Protocol for the Synthesis of Chalcogenated Furans from 1,4-Enediones and Thiols. Org. Biomol. Chem. 2019, 17, 982–991.
- 29(a) Yin, Y.; Ma, W.; Chai, Z.; Zhao, G. Et2Zn-Catalyzed Intramolecular Hydroamination of Alkynyl Sulfonamides and the Related Tandem Cyclization/Addition Reaction. J. Org. Chem. 2007, 72, 5731–5736; (b) Boominathan, S. S. K.; Senadi, G. C.; Vandavasi, J. K.; Chen, J. Y.-F.; Wang, J.-J. An Iron-catalyzed Cascade Approach to Benzo[b]carbazole Synthesis Followed by 1,4-Sulfonyl Migration. Chem.-Eur. J. 2015, 21, 3193–3197; (c) Chong, E.; Blum, S. A. Aminoboration: Addition of B–N σ Bonds Across C–C π Bonds. J. Am. Chem. Soc. 2015, 137, 10144–10147; (d) Liu, J.; Xie, X.; Liu, Y. Silver-catalyzed Cascade Cyclization–Stannylation of o-Alkynylaniline Derivatives with 2-Tributylstannylfuran: An Efficient Synthesis of (3-Indolyl)stannanes. Chem. Commun. 2013, 49, 11794–11796; (e) Zhao, X.; Li, Q.; Xu, J.; Wang, D.; Zhang-Negrerie, D.; Du, Y. Cascade Synthesis of Benzothieno[3,2-b]indoles under Oxidative Conditions Mediated by CuBr and Tert-butyl Hydroperoxide. Org. Lett. 2018, 20, 5933–5937.




