Evolution of Routes for Asymmetric Total Synthesis of Cyclocitrinol Enabled by Type II [5+2] Cycloaddition†
Jianlei Wu
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
‡ These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Junyang Liu
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Academy for Advanced Interdisciplinary Studies, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
‡ These authors contributed equally to this work.
E-mail: [email protected]; [email protected]Search for more papers by this authorJian-Hong Fan
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorZhi-Dong Xie
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Academy for Advanced Interdisciplinary Studies, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorHukun Qin
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Chuang-Chuang Li
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]; [email protected]Search for more papers by this authorJianlei Wu
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
‡ These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Junyang Liu
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Academy for Advanced Interdisciplinary Studies, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
‡ These authors contributed equally to this work.
E-mail: [email protected]; [email protected]Search for more papers by this authorJian-Hong Fan
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorZhi-Dong Xie
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Academy for Advanced Interdisciplinary Studies, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorHukun Qin
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Chuang-Chuang Li
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]; [email protected]Search for more papers by this authorDedicated to Department of Chemistry, SUSTech, on the Occasion of Her 10th Anniversary
Main observation and conclusion
The asymmetric total synthesis of an unusual C25 steroid containing a unique bicyclo[4.4.1]undecene A/B ring system, resulting in the synthesis of cyclocitrinol (1) and its isomer Δ8,14-cyclocitrinol (38), is reported. Initial attempts to construct the synthetically challenging bicyclo[4.4.1]undecene A/B ring system using a type II [5+2] cycloaddition showed that a chiral substituent at the allylic position of the alkene (C6, cyclocitrinol numbering) controlled the stereoselective outcome of the cycloaddition reaction. Late-stage migration of the tetrasubstituted C8–C14 double bond in Δ8,14-cyclocitrinol (38) to obtain cyclocitrinol (1) proved challenging, inspiring an alternative approach. The chiral β-CH2OR group on the allylic substituent at C6 played a pivotal role both in controlling the diastereoselectivity of the type II [5+2] cycloaddition and retaining the C6 substituent under lithium–amine conditions.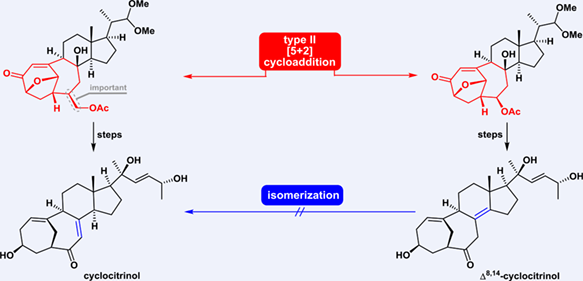
Supporting Information
| Filename | Description |
|---|---|
| cjoc202000698-sup-0001-Supinfo.pdfPDF document, 2.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Zeelen, F. J. In Principles of Medical Biology, Vol. 8, Eds.: Bittar, E. E.; Bittar, N., Elsevier, 1997, pp. 427–463.
- 2(a) Bohl, M.; Duax, W. L. Molecular Structure and Biological Activity of Steroids, CRC Press, 2018; (b) Meher, C. P.; Sethy, S. P.; Pochaiah, B. Structure and biological activities: Steroid moieties. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 253–272; (c) Biellmann, J.-F. Enantiomeric Steroids: Synthesis, Physical, and Biological Properties. Chem. Rev. 2003, 103, 2019–2034.
- 3 Kozlovsky, A. G.; Zhelifonova, V. P.; Ozerskaya, S. M.; Vinokurova, N. G.; Adanin, V. M.; Gräfe, U. Cyclocitrinol, a new fungal metabolite from Penicillium citrinum. Pharmazie 2000, 55, 470–471.
- 4 Amagata, T.; Amagata, A.; Tenney, K.; Valeriote, F. A.; Lobkovsky, E.; Clardy, J.; Crews, P. Unusual C25 steroids produced by a sponge-derived Penicillium citrinum. Org. Lett. 2003, 5, 4393–4396.
- 5(a) Hoang, T. P. T.; Roullier, C.; Genta-Jouve, G.; Boumard, M.-C.; Robiou du Pont, T.; Nazih, H.; Pouchus, Y.-F.; Grovel, O. C25 steroids from the marine mussel-derived fungus Penicillium ubiquetum MMS330. Phytochem. Lett. 2019, 34, 18–24; (b) Yu, F.-X.; Li, Z.; Chen, Y.; Yang, Y.-H.; Li, G.-H.; Zhao, P.-J. Four new steroids from the endophytic fungus Chaetomium sp. M453 derived of Chinese herbal medicine Huperzia serrata. Fitoterapia 2017, 117, 41–46; (c) Li, B.; Wei, W.; Luan, N.; Li, J.; Lao, W.; Zhang, W.; Shang, X. Structure elucidation and NMR assignments of two unusual isomeric aromatic monacolin analogs from Monascus purpureus. Magn. Reson. Chem. 2015, 53, 233–236; (d) Ying, Y.-M.; Zheng, Z.-Z.; Zhang, L.-W.; Shan, W.-G.; Wang, J.-W.; Zhan, Z.-J. Rare C25 Steroids Produced by Penicillium chrysogenum P1X, a Fungal Endophyte of Huperzia serrata. Helv. Chim. Acta 2014, 97, 95–101; (e) Xia, M. W.; Cui, C. B.; Li, C. W.; Wu, C. J. Three new and eleven known unusual C25 steroids: activated production of silent metabolites in a marine-derived fungus by chemical mutagenesis strategy using diethyl sulphate. Mar. Drugs 2014, 12, 1545–1568; (f) Du, L.; Zhu, T.; Fang, Y.; Gu, Q.; Zhu, W. Unusual C25 steroid isomers with bicyclo[4.4.1]A/B rings from a volcano ash-derived fungus Penicillium citrinum. J. Nat. Prod. 2008, 71, 1343–1351; (g) Marinho, A. M. d. R.; Rodrigues-Filho, E.; Ferreira, A. G.; Santos, L. S. C25 steroid epimers produced by Penicillium janthinellum, a fungus isolated from fruits Melia azedarach. J. Braz. Chem. Soc. 2005, 16, 1342–1346.
- 6 Mak, J. Y. W.; Pouwer, R.; Williams, C. M. Natural Products with Anti- Bredt and Bridgehead Double Bonds. Angew. Chem. Int. Ed. 2014, 53, 13664–13688.
- 7 Liu, J.; Liu, X.; Wu, J.; Li, C.-C. Total Synthesis of Natural Products Containing a Bridgehead Double Bond. Chem 2020, 6, 579–615.
- 8(a) El Sheikh, S.; Meier zu Greffen, A.; Lex, J.; Neudörfl, J.; Schmalz, H. Synthesis of the Core Structure of the Cyclocitrinols via SmI2-Mediated Fragmentation of a Cyclopropane Precursor. Synlett 2007, 1881–1884; (b) Plummer, C. W.; Soheili, A.; Leighton, J. L. A Tandem Cross-Metathesis/Semipinacol Rearrangement Reaction. Org. Lett. 2012, 14, 2462–2464; (c) Liu, T.; Yan, Y. B.; Ma, H. Y.; Ding, K. Synthesis of Cyclocitrinol Skeleton via a Carbocation Rearrangement. Chin. J. Org. Chem. 2014, 34, 1793–1799; (d) Plummer, C. W.; Wei, C. S.; Yozwiak, C. E.; Soheili, A.; Smithback, S. O.; Leighton, J. L. Design, development, mechanistic elucidation, and rational optimization of a tandem Ireland Claisen/Cope rearrangement reaction for rapid access to the (iso)cyclocitrinol core. J. Am. Chem. Soc. 2014, 136, 9878–9881; (e) Plummer, C. W.; Wei, C. S.; Yozwiak, C. E.; Soheili, A.; Smithback, S. O.; Leighton, J. L. Correction to "Design, Development, Mechanistic Elucidation, and Rational Optimization of a Tandem Ireland Claisen/Cope Rearrangement Reaction for Rapid Access to the (Iso)Cyclocitrinol Core". J. Am. Chem. Soc. 2015, 137, 13722.
- 9 Liu, J.; Wu, J.; Fan, J. H.; Yan, X.; Mei, G.; Li, C.-C. Asymmetric Total Synthesis of Cyclocitrinol. J. Am. Chem. Soc. 2018, 140, 5365–5369.
- 10(a) Wang, Y.; Ju, W.; Tian, H.; Tian, W.; Gui, J. Scalable Synthesis of Cyclocitrinol. J. Am. Chem. Soc. 2018, 140, 9413–9416; (b) Wang, Y.; Ju, W.; Tian, H.; Sun, S.; Li, X.; Tian, W.; Gui, J. Facile Access to Bridged Ring Systems via Point-to-Planar Chirality Transfer: Unified Synthesis of Ten Cyclocitrinols. J. Am. Chem. Soc. 2019, 141, 5021–5033.
- 11(a) Zhang, W.; Zhou, Z.-X.; Zhu, X.-J.; Sun, Z.-H.; Dai, W.-M.; Li, C.-C. Asymmetric Total Synthesis of the Highly Strained 4β-Acetoxyprobotryane-9β,15α-diol. J. Am. Chem. Soc. 2020, 142, 19868–19873; (b) Cheng, M.-J.; Zhong, L.-P.; Gu, C.-C.; Zhu, X.-J.; Chen, B.; Liu, J.-S.; Wang, L.; Ye, W.-C.; Li, C.-C. Asymmetric Total Synthesis of Bufospirostenin A. J. Am. Chem. Soc. 2020, 142, 12602–12607; (c) Tong, Z.; Ding, H. Asymmetric Total Synthesis of Spirostanol Bufospirostenin A. Chin. J. Org. Chem. 2020, 40, 3984–3985; (d) Cheng, M.-J.; Cao, J.-Q.; Yang, X.-Y.; Zhong, L.-P.; Hu, L.-J.; Lu, X.; Hou, B.-L.; Hu, Y.-J.; Wang, Y.; You, X.-F.; Wang, L.; Ye, W.-C.; Li, C.-C. Catalytic asymmetric total syntheses of myrtucommuacetalone, myrtucommuacetalone B, and callistrilones A, C, D and E. Chem. Sci. 2018, 9, 1488–1495; (e) Chen, B.; Liu, X.; Hu, Y.-J.; Zhang, D.-M.; Deng, L.; Lu, J.; Min, L.; Ye, W.-C.; Li, C.-C. Enantioselective total synthesis of (−)-colchicine, (+)-demecolcinone and metacolchicine: determination of the absolute configurations of the latter two alkaloids. Chem. Sci. 2017, 8, 4961–4966; (f) Han, J.-C.; Li, F.; Li, C.-C. Collective Synthesis of Humulanolides Using a Metathesis Cascade Reaction. J. Am. Chem. Soc. 2014, 136, 13610–13613.
- 12For type II oxidopyrylium ylide [5+2] cycloadditon and its application in total synthesis, see: (a) Mei, G.; Liu, X.; Qiao, C.; Chen, W.; Li, C.-C. Type II intramolecular [5+2] cycloaddition: facile synthesis of highly functionalized bridged ring systems. Angew. Chem. Int. Ed. 2015, 54, 1754–1758; (b) Min, L.; Liu, X.; Li, C.-C. Total Synthesis of Natural Products with Bridged Bicyclo[m.n.1] Ring Systems via Type II [5 + 2] Cycloaddition. Acc. Chem. Res. 2020, 53, 703–718; (c) Min, L.; Lin, X.; Li, C.-C. Asymmetric Total Synthesis of (−)-Vinigrol. J. Am. Chem. Soc. 2019, 141, 15773–15778; (d) Liu, X.; Liu, J.; Wu, J.; Huang, G.; Liang, R.; Chung, L.; Li, C.-C. Asymmetric Total Synthesis of Cerorubenic Acid-III. J. Am. Chem. Soc. 2019, 141, 2872–2877.
- 13For type I oxidopyrylium ylide [5+2] cycloadditon and its application in total synthesis, see: (a) Wender, P. A.; Rice, K. D.; Schnute, M. E. The First Formal Asymmetric Synthesis of Phorbol. J. Am. Chem. Soc. 1997, 119, 7897–7898; (b) Zhang, M.; Liu, N.; Tang, W. Stereoselective Total Synthesis of Hainanolidol and Harringtonolide via Oxidopyrylium-Based [5 + 2] Cycloaddition. J. Am. Chem. Soc. 2013, 135, 12434–12438; (c) Krüger, S.; Gaich, T. Enantioselective, Protecting- Group-Free Total Synthesis of Sarpagine Alkaloids—A Generalized Approach. Angew. Chem. Int. Ed. 2015, 54, 315–317; (d) Wender, P. A.; Jesudason, C. D.; Nakahira, H.; Tamura, N.; Tebbe, A. L.; Ueno, Y. The First Synthesis of a Daphnane Diterpene: The Enantiocontrolled Total Synthesis of (+)-Resiniferatoxin. J. Am. Chem. Soc. 1997, 119, 12976–12977; (e) Wender, P. A.; Lee, H. Y.; Wilhelm, R. S.; Williams, P. D. Studies on tumor promoters. 7. The synthesis of a potentially general precursor of the tiglianes, daphnanes, and ingenanes. J. Am. Chem. Soc. 1989, 111, 8954–8957; (f) Wender, P. A.; Kogen, H.; Lee, H. Y.; Munger, J. D.; Wilhelm, R. S.; Williams, P. D. Studies on tumor promoters. 8. The synthesis of phorbol. J. Am. Chem. Soc. 1989, 111, 8957–8958; (g) Mei, G.; Yuan, H.; Gu, Y.; Chen, W.; Chung, L. W.; Li, C.-C. Dearomative Indole [5+2] Cycloaddition Reactions: Stereoselective Synthesis of Highly Functionalized Cyclohepta[b]indoles. Angew. Chem. Int. Ed. 2014, 53, 11051–11055; For selected reviews on oxidopyrylium ylide [5+2] cycloadditon, see: (h) Gao, K.; Zhang, Y.-G.; Wang, Z.; Ding, H. Recent development on the [5+2] cycloadditions and their application in natural product synthesis. Chem. Commun. 2019, 55, 1859–1878; (i) Liu, X.; Hu, Y.-J.; Fan, J.-H.; Zhao, J.; Li, S.; Li, C.-C. Recent synthetic studies towards natural products via [5 + 2] cycloaddition reactions. Org. Chem. Front. 2018, 5, 1217–1228; (j) Bejcek, L. P.; Murelli, R. P. Oxidopyrylium [5+2] cycloaddition chemistry: Historical perspective and recent advances (2008–2018). Tetrahedron 2018, 74, 2501–2521; (k) Ylijoki, K. E. O.; Stryker, J. M. [5 + 2] Cycloaddition Reactions in Organic and Natural Product Synthesis. Chem. Rev. 2013, 113, 2244–2266.
- 14(a) Mack, D. J.; Guo, B.; Njardarson, J. T. Synthesis of allylic and homoallylic alcohols from unsaturated cyclic ethers using a mild and selective C–O reduction approach. Chem. Commun. 2012, 48, 7844–7846; (b) Reddy, C. R.; Reddy, G. B.; Rao, C. L. Titanium tetrachloride mediated reductive ring opening of C-aryl pseudoglycals. Tetrahedron Lett. 2008, 49, 863–866; (c) Qin, H.-L.; Lowe, J. T.; Panek, J. S. Mild Reductive Opening of Aryl Pyranosides Promoted by Scandium(III) Triflate. J. Am. Chem. Soc. 2007, 129, 38–39; (d) Molander, G. A.; Eastwood, P. R. Total Synthesis of (+)-Dactylol via a Novel [3 + 5] Annulation Approach. J. Org. Chem. 1995, 60, 4559–4565; (e) Zhou, J.; Lu, G.; Huang, X.; Wu, S. Stereoselective Synthesis of Alkenyl Alcohols Using Dissolving Metal (Ca,Na) Reduction. Synth. Commun. 1991, 21, 435–441; (f) Rigby, J. H.; Wilson, J. Z. Total synthesis of guaianolides: (±)-dehydrocostus lactone and (±)-estafiatin. J. Am. Chem. Soc. 1984, 106, 8217–8224; (g) Krishnamurthy, S.; Brown, H. C. Selective reductions. 25. Remarkably facile reductive opening of cyclic ethers by the lithium tri-tert-butoxyaluminohydride-triethylborane combination. J. Org. Chem. 1979, 44, 3678–3682; (h) Maercker, A. Ether Cleavage with Organo-Alkali-Metal Compounds and Alkali Metals. Angew. Chem. Int. Ed. Engl. 1987, 26, 972–989; (i) Bhatt, M. V.; Kulkarni, S. U. Cleavage of Ethers. Synthesis 1983, 249–282.
- 15(a) Shipilovskikh, S. A.; Rubtsov, A. E.; Malkov, A. V. Oxidative Dehomologation of Aldehydes with Oxygen as a Terminal Oxidant. Org. Lett. 2017, 19, 6760–6762;
(b) Kipke, A.; Schöning, K.-U.; Yusubov, M.; Kirschning, A. TEMPO-Mediated Oxidative Deformylation of Aldehydes: Applications in the Synthesis of Polyketide Fragments. Eur. J. Org. Chem. 2017, 6906–6913;
(c) Watanabe, E.; Kaiho, A.; Kusama, H.; Iwasawa, N. Cobalt–Salen Complex-Catalyzed Oxidative Generation of Alkyl Radicals from Aldehydes for the Preparation of Hydroperoxides. J. Am. Chem. Soc. 2013, 135, 11744–11747;
(d) Sun, H.; Yang, C. H.; Gao, F.; Li, Z.; Xia, W. Oxidative C–C Bond Cleavage of Aldehydes via Visible-Light Photoredox Catalysis. Org. Lett. 2013, 15, 624–627;
(e) Tiwari, B.; Zhang, J.; Chi, Y. R. Facile Access to Chiral Ketones through Metal-Free Oxidative C—C Bond Cleavage of Aldehydes by O2. Angew. Chem. Int. Ed. 2012, 51, 1911–1914;
(f) Havare, N.; Plattner, D. A. Oxidative Cleavage of α-Aryl Aldehydes Using Iodosylbenzene. Org. Lett. 2012, 14, 5078–5081;
(g) Van Rheenen, V. Copper-catalyzed oxygenation of branched aldehydes – an efficient ketone synthesis. Tetrahedron Lett. 1969, 10, 985–988.
10.1016/S0040-4039(01)97716-0 Google Scholar
- 16 Liu, X.; Liu, J.; Zhao, J.; Li, S.; Li, C.-C. Toward the Total Synthesis of Eurifoloid A. Org. Lett. 2017, 19, 2742–2745.
- 17Compound 12: (1R,3aR,7aR)-1-[(1S)-2,2-dimethoxy-1-methylethyl]octahydro-7a-methyl-4H-inden-4-one, CAS#: 957214–01–6, was purchased in 500 g for $4700 from Shanghai Huqi Pharmaceutical Science & Technology Co. Ltd. in 2016. Compound 12 also could be synthesized from vitamin D2 in one step, see: Tavera-Mendoza, L. E.; Quach, T. D.; Dabbas, B.; Hudon, J.; Liao, X.; Palijan, A.; Gleason, J. L.; White, J. H. Incorporation of histone deacetylase inhibition into the structure of a nuclear receptor agonist. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 8250–8255.
- 18 Angeles, A. R.; Waters, S. P.; Danishefsky, S. J. Total Syntheses of (+)- and (−)-Peribysin E. J. Am. Chem. Soc. 2008, 130, 13765–13770.
- 19 Allred, G. D.; Liebeskind, L. S. Copper-Mediated Cross-Coupling of Organostannanes with Organic Iodides at or below Room Temperature. J. Am. Chem. Soc. 1996, 118, 2748–2749.
- 20 Dhawan, D.; Grover, S. K. Facile Reduction of Chalcones to Dihydrochalcones with NaBH4/Ni2+ System. Synth. Commun. 1992, 22, 2405–2409.
- 21(a) Lumbroso, A.; Koschker, P.; Vautravers, N. R.; Breit, B. Redox- Neutral Atom-Economic Rhodium-Catalyzed Coupling of Terminal Alkynes with Carboxylic Acids Toward Branched Allylic Esters. J. Am. Chem. Soc. 2011, 133, 2386–2389; (b) Shing, T. K. M.; Yeung, Y.-Y.; Su, P. L. Mild Manganese(III) Acetate Catalyzed Allylic Oxidation: Application to Simple and Complex Alkenes. Org. Lett. 2006, 8, 3149–3151; (c) Delcamp, J. H.; White, M. C. Sequential Hydrocarbon Functionalization: Allylic C−H Oxidation/Vinylic C−H Arylation. J. Am. Chem. Soc. 2006, 128, 15076–15077; (d) Chen, M. S.; Prabagaran, N.; Labenz, N. A.; White, M. C. Serial Ligand Catalysis: A Highly Selective Allylic C−H Oxidation. J. Am. Chem. Soc. 2005, 127, 6970–6971; (e) Chen, M. S.; White, M. C. A Sulfoxide-Promoted, Catalytic Method for the Regioselective Synthesis of Allylic Acetates from Monosubstituted Olefins via C−H Oxidation. J. Am. Chem. Soc. 2004, 126, 1346–1347; (f) Yu, J.-Q.; Corey, E. J. A Mild, Catalytic, and Highly Selective Method for the Oxidation of α,β-Enones to 1,4-Enediones. J. Am. Chem. Soc. 2003, 125, 3232–3233.
- 22 Kolb, H. C.; VanNieuwenhze, M. l. S.; Sharpless, K. B. Catalytic Asymmetric Dihydroxylation. Chem. Rev. 1994, 94, 2483–2547.
- 23 Alcaraz, L.; Harnett, J. J.; Mioskowski, C.; Martel, J. P.; Le Gall, T.; Shin, D.-S.; Falck, J. R. Novel conversion of epoxides to one carbon homologated allylic alcohols by dimethylsulfonium methylide. Tetrahedron Lett. 1994, 35, 5449–5452.
- 24(a) Schindler, C. S.; Carreira, E. M. Rapid formation of complexity in the total synthesis of natural products enabled by oxabicyclo[2.2.1]heptene building blocks. Chem. Soc. Rev. 2009, 38, 3222–3241; (b) Rayabarapu, D. K.; Cheng, C.-H. New Catalytic Reactions of Oxa- and Azabicyclic Alkenes. Acc. Chem. Res. 2007, 40, 971–983; (c) Gómez Arrayás, R.; Cabrera, S.; Carretero, J. C. s. Copper-Catalyzed Ring- Opening of Heterobicyclic Alkenes with Grignard Reagents: Remarkably High anti-Stereocontrol. Synthesis 2006, 1205–1219; (d) Lautens, M.; Fagnou, K.; Hiebert, S. Transition Metal-Catalyzed Enantioselective Ring-Opening Reactions of Oxabicyclic Alkenes. Acc. Chem. Res. 2003, 36, 48–58; (e) Chiu, P.; Lautens, M. In II Stereoselective Heterocyclic Synthesis, Ed.: Metz, P., Springer Berlin Heidelberg, Berlin, Heidelberg, 1997, pp. 1–85.
- 25(a) Oblak, E. Z.; VanHeyst, M. D.; Li, J.; Wiemer, A. J.; Wright, D. L. Cyclopropene Cycloadditions with Annulated Furans: Total Synthesis of (+)- and (−)-Frondosin B and (+)-Frondosin A. J. Am. Chem. Soc. 2014, 136, 4309–4315;
(b) Williams, Y. D.; Meck, C.; Mohd, N.; Murelli, R. P. Triflic Acid-Mediated Rearrangements of 3-Methoxy-8-oxabicyclo[3.2.1]octa-3,6-dien-2-ones: Synthesis of Methoxytropolones and Furans. J. Org. Chem. 2013, 78, 11707–11713;
(c) Xing, S.; Li, Y. L.; Li, Z.; Liu, C.; Ren, J.; Wang, Z. Lewis Acid Catalyzed Intramolecular [3+2] Cross-Cycloaddition of Donor–Acceptor Cyclopropanes with Carbonyls: A General Strategy for the Construction of Acetal[n.2.1] Skeletons. Angew. Chem. Int. Ed. 2011, 50, 12605–12609;
(d) Murali Krishna, U.; Trivedi, G. K. Studies towards the synthesis of FCRR toxin: an expeditious entry into 7–5–6 ring systems via [5+2] oxidopyrylium-alkene cycloaddition. Tetrahedron Lett. 2004, 45, 257–259;
(e) Hodgson, D. M.; Maxwell, C. R.; Miles, T. J.; Paruch, E.; Stent, M. A. H.; Matthews, I. R.; Wilson, F. X.; Witherington, J. Enantioselective Alkylative Double Ring Opening of Epoxides: Synthesis of Enantioenriched Unsaturated Diols and Amino Alcohols. Angew. Chem. Int. Ed. 2002, 41, 4313–4316;
10.1002/1521-3773(20021115)41:22<4313::AID-ANIE4313>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar(f) Lee, J. C.; Cha, J. K. Total Synthesis of Tropoloisoquinolines: Imerubrine, Isoimerubrine, and Grandirubrine1. J. Am. Chem. Soc. 2001, 123, 3243–3246; (g) Lautens, M.; Hiebert, S.; Renaud, J.-L. Mechanistic Studies of the Palladium-Catalyzed Ring Opening of Oxabicyclic Alkenes with Dialkylzinc. J. Am. Chem. Soc. 2001, 123, 6834–6839; (h) Rodríguez, J. R.; Castedo, L.; Mascareñas, J. Tandem Organolithium Addition/Oxa-Bridge Opening of 8-Oxa[3.2.1]bicyclic Pyrone-Alkene Adducts. Synthesis 2000, 980–984; (i) Kreiselmeier, G.; Föhlisch, B. Total synthesis of racemic lasidiol via intramolecular [4+3] cycloaddition. Tetrahedron Lett. 2000, 41, 1375–1379; (j) Lautens, M.; Fillion, E.; Sampat, M. Base-Induced Ring Opening of Aza- and Thiaoxa[3.2.1] and -[3.3.1]bicycles as an Enantioselective Approach to Azepines, Thiepines, and Thiocines. J. Org. Chem. 1997, 62, 7080–7081.
- 26 Barton, D. H. R.; McCombie, S. W. A new method for the deoxygenation of secondary alcohols. J. Chem. Soc., Perkin Trans. 1 1975, 1574–1585.
- 27Alternative condition for dehydration of 28 to improve the selectivity was found, see the supporting information for detail.
- 28 Han, J. H.; Kwon, Y. E.; Sohn, J.-H.; Ryu, D. H. A facile method for the rapid and selective deprotection of methoxymethyl (MOM) ethers. Tetrahedron 2010, 66, 1673–1677.
- 29 Lee, J.; Deng, L. Asymmetric Approach toward Chiral Cyclohex-2- enones from Anisoles via an Enantioselective Isomerization by a New Chiral Diamine Catalyst. J. Am. Chem. Soc. 2012, 134, 18209–18212.
- 30 Einhorn, J.; Einhorn, C.; Ratajczak, F.; Pierre, J.-L. Efficient and Highly Selective Oxidation of Primary Alcohols to Aldehydes by N-Chlorosuccinimide Mediated by Oxoammonium Salts. J. Org. Chem. 1996, 61, 7452–7454.




