Deoxyfluorination of Carboxylic, Sulfonic, Phosphinic Acids and Phosphine Oxides by Perfluoroalkyl Ether Carboxylic Acids Featuring CF2O Units
Shiyu Zhao
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Yong Guo
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected]; [email protected]Search for more papers by this authorZhaoben Su
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorChengying Wu
Sanming Hexafluo Chemicals Company, Ltd., Fluorinated New Material Industry Park, Mingxi, Sanming, Fujian, 365200 China
Search for more papers by this authorWei Chen
Sanming Hexafluo Chemicals Company, Ltd., Fluorinated New Material Industry Park, Mingxi, Sanming, Fujian, 365200 China
Search for more papers by this authorCorresponding Author
Qing-Yun Chen
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected]; [email protected]Search for more papers by this authorShiyu Zhao
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Yong Guo
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected]; [email protected]Search for more papers by this authorZhaoben Su
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorChengying Wu
Sanming Hexafluo Chemicals Company, Ltd., Fluorinated New Material Industry Park, Mingxi, Sanming, Fujian, 365200 China
Search for more papers by this authorWei Chen
Sanming Hexafluo Chemicals Company, Ltd., Fluorinated New Material Industry Park, Mingxi, Sanming, Fujian, 365200 China
Search for more papers by this authorCorresponding Author
Qing-Yun Chen
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected]; [email protected]Search for more papers by this authorMain observation and conclusion
The deoxyfluorination of carboxylic, sulfonic, phosphinic acids and phosphine oxides is a fundamentally important approach to access acyl fluorides, sulfonyl fluorides and phosphoric fluorides, thus the development of inexpensive, stable, easy-to-handle, versatile, and efficient deoxyfluorination reagents is highly desired. Herein, we report the use of potassium salts of perfluoroalkyl ether carboxylic acids (PFECA) featuring CF2O units as deoxyfluorination reagents, which are generated mainly as by-products in the manufacture of hexafluoropropene oxide (HFPO). The synthesis of acyl fluorides, sulfonyl fluorides and phosphoric fluorides can be realized via carbonic difluoride (COF2) generated in situ from thermal degradation of the PFECA salt.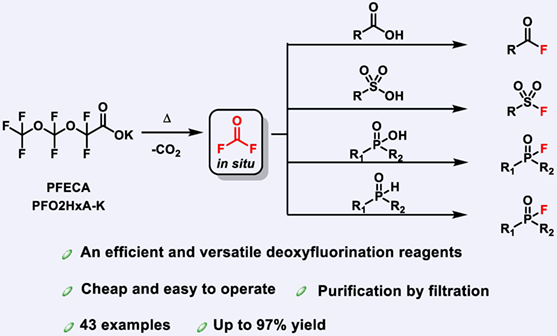
Supporting Information
| Filename | Description |
|---|---|
| cjoc202000662-sup-0001-Supinfo.pdfPDF document, 5.4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Ogiwara, Y.; Sakai, N. Acyl Fluorides in Late-Transition-Metal Catalysis. Angew. Chem. Int. Ed. 2020, 59, 574–594; (b) Blanchard, N.; Bizet, V. Acid Fluorides in Transition-Metal Catalysis: A Good Balance between Stability and Reactivity. Angew. Chem. Int. Ed. 2019, 58, 2–6; (c) Gonay, M.; Batisse, C.; Paquin, J. F. Recent Advances in the Synthesis of Acyl Fluorides. Synthesis 2021, 53, 653–665.
- 2(a) Dong, J.; Krasnova, L.; Finn, M. G.; Sharpless, K. B. Sulfur(VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry. Angew. Chem. Int. Ed. 2014, 53, 9430–9448; (b) Barrow, A. S.; Smedley, C. J.; Zheng, Q.; Li, S.; Dong, J.; Moses, J. E. The Growing applications of SuFEx Click Chemistry. Chem. Soc. Rev. 2019, 48, 4731–4758; (c) Xu, L.; Dong, J. Click Chemistry: Evolving on the Fringe. Chin. J. Chem. 2020, 38, 414–419.
- 3(a) El Sayed, S.; Pascual, L.; Agostini, A., Martínez-Máñez, R.; Sancenón, F.; Costero, A. M.; Parra, M.; Gil, S. A chromogenic probe for the selective recognition of sarin and soman mimic DFP. ChemistryOpen 2014, 3, 142–145; (b) Barba-Bon, A.; Costero, A. M.; Gil, S.; Martínez-Máñez, R.; Sancenón, F. Selective chromo-fluorogenic detection of DFP (a Sarin and Soman mimic) and DCNP (a Tabun mimic) with a unique probe based on a boron dipyrromethene (BODIPY) dye. Org. Biomol. Chem. 2014, 12, 8745–8751.
- 4(a) Boreux, A.; Indukuri, K.; Gagosz, F.; Riant, O. Acyl fluorides as efficient electrophiles for the copper-catalyzed boroacylation of allenes. ACS Catal. 2017, 7, 8200–8204; (b) Ogiwara, Y.; Sakino, D.; Sakurai, Y.; Sakai, N. Acid fluorides as acyl electrophiles in Suzuki-Miyaura coupling. Eur. J. Org. Chem. 2017, 2017, 4324–4327; (c) Malapit, C. A.; Bour, J. R.; Brigham, C. E.; Sanford, M. S. Base-free nickel-catalysed decarbonylative Suzuki-Miyaura coupling of acid fluorides. Nature 2018, 563, 100–104; (d) Malapit, C. A.; Bour, J. R.; Laursen, S. R.; Sanford, M. S. Mechanism and scope of nickel-catalyzed decarbonylative borylation of carboxylic acid fluorides. J. Am. Chem. Soc. 2019, 141, 17322–17330; (e) Wang, J.; Hoerrner, M. E.; Watson, M. P.; Weix, D. J. Nickel-Catalyzed Synthesis of Dialkyl Ketones from the Coupling of N-Alkyl Pyridinium Salts with Activated Carboxylic Acids. Angew. Chem. Int. Ed. 2020, 59, 13484–13489; (f) Fu, L.; Chen, Q.; Wang, Z.; Nishihara, Y. Palladium-Catalyzed Decarbonylative Alkylation of Acyl Fluorides. Org. Lett. 2020, 22, 2350–2353.
- 5(a) Ichihara, J.; Matsuo, T.; Hanafusa, T.; Ando, T. The combination of potassium fluoride and calcium fluoride: a useful heterogeneous fluorinating reagent. J. Chem. Soc., Chem. Commun. 1986, 793–794; (b) Chworoś, A.; Woźniak, L. A. A facile conversion of thio- and selenophosphoric acids and their derivatives into fluoridates by means of reaction with silver fluoride. Tetrahedron Lett. 1999, 40, 9337–9340; (c) Matesic, L.; Wyatt, N. A.; Fraser, B. H.; Roberts, M. P.; Pham, T. Q.; Greguric, I. Ascertaining the suitability of aryl sulfonyl fluorides for [18F] radiochemistry applications: a systematic investigation using microfluidics. J. Org. Chem. 2013, 78, 11262–11270; (d) Tang, L.; Yang, Y.; Wen, L.; Yang, X.; Wang, Z. Catalyst-free radical fluorination of sulfonyl hydrazides in water. Green Chem. 2016, 18, 1224–1228; (e) Jiang, Y.; Alharbi, N. S.; Sun, B.; Qin, H.-L. Facile One-Pot Synthesis of Sulfonyl Fluorides from Sulfonates or Sulfonic Acids. RSC Adv. 2019, 9, 13863–13867.
- 6For selected examples: (a) Middleton, W. J. New fluorinating reagents. Dialkylaminosulfur fluorides. J. Org. Chem. 1975, 40, 574–578; (b) Takaoka, A.; Iwakiri, H.; Ishikawa, N. F-propene-dialkylamine reaction products as fluorinating agents. Bull. Chem. Soc. Jpn. 1979, 52, 3377–3380; (c) Carpino, L. A.; Sadat-Aalaee, D.; Chao, H. G.; DeSelms, R. H. ((9-Fluorenylmethyl)oxy)carbonyl (FMOC) amino acid fluorides. Convienient new peptide coupling reagents applicable to the FMOC/tert-butyl strategy for solution and solid-phase syntheses. J. Am. Chem. Soc. 1990, 112, 9651–9652; (d) Carpino, L. A.; El-Faham, A. Tetramethylfluoroformamidinium hexafluorophosphate: a rapid- acting peptide coupling reagent for solution and solid phase peptide synthesis. J. Am. Chem. Soc. 1995, 117, 5401–5402; (e) Lal, G. S.; Pez, G. P.; Pesaresi, R. J.; Prozonic, F. M.; Cheng, H. Bis(2-methoxyethyl) aminosulfur trifluoride: a new broad-spectrum deoxofluorinating agent with enhanced thermal stability. J. Org. Chem. 1999, 64, 7048–7054; (f) Beaulieu, F.; Beauregard, L.-P.; Courchesne, G.; Couturier, M.; LaFlamme, F.; L'Heureux, A. Aminodifluorosulfinium tetrafluoroborate salts as stable and crystalline deoxofluorinating reagents. Org. Lett. 2009, 11, 5050–5053; (g) Scattolin, T.; Deckers, K.; Schoenebeck, F. Direct synthesis of acyl fluorides from carboxylic acids with the bench-stable solid reagent (Me4N)SCF3. Org. Lett. 2017, 19, 5740–5743; (h) Yang, Z.; Chen, S.; Yang, F.; Zhang, C.; Dou, Y.; Zhou, Q.; Yan, Y.; Tang, L. PPh3/Selectfluor-Mediated Transformation of Carboxylic Acids into Acid Anhydrides and Acyl Fluorides and Its Application in Amide and Ester Synthesis. Eur. J. Org. Chem. 2019, 2019, 5998–6002; (i) Munoz, S. B.; Dang, H.; Ispizua-Rodriguez, X.; Mathew, T.; Prakash, G. K. S. Direct access to acyl fluorides from carboxylic acids using a phosphine/fluoride deoxyfluorination reagent system. Org. Lett. 2019, 21, 1659–1663; (j) Gonay, M.; Batisse, C.; Paquin, J. F. Synthesis of Acyl Fluorides from Carboxylic Acids Using NaF-Assisted Deoxofluorination with XtalFluor-E. J. Org. Chem. 2020, 85, 10253–10260; (k) Foth, P. J.; Malig, T. C.; Yu, H.; Bolduc, T. G.; Hein, J. E.; Sammis, G. M. Halide-Accelerated Acyl Fluoride Formation Using Sulfuryl Fluoride. Org. Lett. 2020, 22, 6682–6686; (l) Le, B.; Wu, H.; Hu, X.; Zhou, X.; Guo, Y.; Chen, Q.-Y.; Liu, C. Rapid Synthesis of Acyl Fluorides from Carboxylic Acids with Cu(O2CCF2SO2F)2. Tetrahedron Lett. 2020, 61, 152624; (m) Song, H.-X.; Tian, Z.-Y.; Xiao, J.-C.; Zhang, C.-P. Tertiary-Amine-Initiated Synthesis of Acyl Fluorides from Carboxylic Acids and CF3SO2OCF3. Chem.-Eur. J. 2020, 26, 16261–16265.
- 7For selected examples: (a) Hasek, W. R.; Smith, W. C.; Engelhardt, V. A. The Chemistry of Sulfur Tetrafluoride. II. The Fluorination of Organic Carbonyl Compounds. J. Am. Chem. Soc. 1960, 82, 543–551; (b) Petrov, V. A.; Swearingen, S.; Hong, W.; Petersen, W. C. 1,1,2,2-Tetrafluoroethyl-N,N-dimethylamine: a new selective fluorinating agent. J. Fluorine Chem. 2001, 109, 25–31; (c) Liu, Y.; Wu, H.; Guo, Y.; Xiao, J.-C.; Chen, Q.-Y.; Liu, C. Trifluoromethylfluorosulfonylation of Unactivated Alkenes Using Readily Available Ag(O2CCF2SO2F) and N-Fluorobenzenesulfonimide. Angew. Chem. Int. Ed. 2017, 56, 15432−15435; (d) Liu, Y.; Lin, Q.; Xiao, Z.; Zheng, C.; Guo, Y.; Chen, Q.-Y.; Liu, C. Zinc-Mediated Intermolecular Reductive Radical Fluoroalkylsulfination of Unsaturated Carbon-Carbon Bonds with Fluoroalkyl Bromides and Sulfur Dioxide. Chem.-Eur. J. 2019, 25, 1824–1828; (e) Lin, Q.; Liu, Y.; Xiao, Z.; Zheng, L.; Zhou, X.; Guo, Y.; Chen, Q.-Y.; Zheng, C.; Liu, C. Intermolecular Oxidative Radical Fluoroalkylfluorosulfonylation of Unactivated Alkenes with (Fluoroalkyl)trimethylsilane, Silver Fluoride, Sulfur Dioxide and N-fluorobenzenesulfonimide. Org. Chem. Front. 2019, 6, 447−450; (f) Liu, Y.; Yu, D.; Guo, Y.; Xiao, J.-C.; Chen, Q.-Y.; Liu, C. Arenesulfonyl Fluoride Synthesis via Copper-Catalyzed Fluorosulfonylation of Arenediazonium Salts. Org. Lett. 2020, 22, 2281−2286; (g) Lin, Q.; Ma, Z.; Zheng, C.; Hu, X. J.; Guo, Y.; Chen, Q.-Y.; Liu, C. Arenesulfonyl fluoride synthesis via copper- free Sandmeyer-type fluorosulfonylation of arenediazonium salts. Chin. J. Chem. 2020, 38, 1107–1110.
- 8For selected examples: (a) Markovskij, L. N.; Pashinnik, V. E.; Kirsanov, A. V. Application of dialkylaminosulfur trifluorides in the synthesis of fluoroorganic compounds. Synthesis 1973, 1973, 787–789; (b) Łopusiński, A.; Michalski, J. Novel Application of Sulfonyl Chloride Fluoride in the Synthesis of Organophosphorus Fluorine Compounds: Direct Conversion of –P(O)H- and –P(O)OH- Groups into –P(O)F- Groups. Angew. Chem. Int. Ed. 1982, 21, 294–294; (c) Konieczko, W. T.; Łopusiński, A.; Michalski, J. Communication thionyl fluoride: a reagent for transformation of diorganyl phosphates and their structural analogues into the fluoridates. Phosphorus, Sulfur Silicon Relat. Elem. 1989, 42, 103–104; (d) Lermontov, S. A.; Popov, A. V.; Zavorin, S. I.; Sukhojenko, I. I.; Kuryleva, N. V.; Martynov, I. V.; Stang, P. Fluorination of phosphorus(+3) derivatives by xenon difluoride. J. Fluorine Chem. 1994, 66, 233–235; (e) Gupta, A. K.; Acharya, J.; Dubey, D. K.; Kaushik, M. P. Dichlorodimethylhydantoin–KF as an efficient reagent for one pot synthesis of dialkylfluorophosphates from dialkylphosphites. J. Fluorine Chem. 2008, 129, 226–229; (f) Wärme, R.; Juhlin, L. A new microscale method for the conversion of phosphorus oxyacids to their fluorinated analogues, using cyanuric fluoride in solution and on solid support. Phosphorus, Sulfur Silicon 2010, 185, 2402–2408; (g) Liu, N.; Mao, L.-L.; Yang, B.; Yang, S.-D. Copper- promoted oxidative-fluorination of arylphosphine under mild conditions. Chem. Commun. 2014, 50, 10879–10882; (h) Purohit, A. K.; Pardasani, D.; Kumar, A.; Goud, D. R.; Jain, R.; Dubey, D. K. A single- step one pot synthesis of dialkyl fluorophosphates from dialkylphosphites. Tetrahedron Lett. 2015, 56, 4593–4595 ; (i) Chen, Q.; Zeng, J.; Yan, X.; Huang, Y.; Wen, C.; Liu, X.; Zhang, K. Electrophilic Fluorination of Secondary Phosphine Oxides and Its Application to P–O Bond Construction. J. Org. Chem. 2016, 81, 10043–10048; (j) Eljo, J.; Murphy, G. K. Direct, oxidative halogenation of diaryl- or dialkylphosphine oxides with (dihaloiodo) arenes. Tetrahedron Lett. 2018, 59, 2965–2969; (k) Bornemann, D.; Pitts, C. R.; Wettstein, L.; Brüning, F.; Küng, S.; Guan, L.; Togni, A. Deoxygenative Fluorination of Phosphine Oxides: A General Route to Fluorinated Organophosphorus(V) Compounds and Beyond. Angew. Chem. Int. Ed. 2020, 59, 22790–22795.
- 9(a) Dams, R. J.; Terrazas, M. S.; Hintzer, K.; Qiu, Z.-M.; Guerra, M. A.; Maurer, A. R.; Kaspar, H.; Lochhaas, K. H.; Juergens, M.; Zipplies, T. C.; Schwertfeger, W. Fluorinated ether compounds and methods of using the same. CN 102149674, 2011; (b) Takagi, H.; Seki, R. Fluorine- containing compound purificaition method. EP 2298726, 2011; (c) Clark, K. P. Fluorochemical foam stabilizers and film formers. US 5750043, 1998; (d) Du, F.; Guo, Y.; Huang, M.; Chen, Q.; Yang, H.; Xie, W.; Cao, W.; Wu, C.; Wang, M. Gemini cationic surfactants with flexible perfluorinated-ether chains. J. Fluorine Chem. 2020, 239, 108632.
- 10Palmer, K. W.; Resnick, P. R. Trifluoromethylation process. US 5475165, 1995.
- 11Zhao, S.; Guo, Y.; Su, Z.; Cao, W.; Wu, C.; Chen, Q.-Y. A Series of Deoxyfluorination Reagents Featuring OCF2 Functional Groups. Org. Lett. 2020, 22, 8634–8637.
- 12O'Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319.




