Monoterpenoid Indole Alkaloids with Promoting Neurite Growth from Tabernaemontana divaricata
Yan Deng
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
These authors contributed to this work equally.
Search for more papers by this authorYang Yu
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
These authors contributed to this work equally.
Search for more papers by this authorBao-Bao Shi
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorMei-Fen Bao
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorSi-Meng Zhao
iHuman Institute, ShanghaiTech University, Shanghai, 200031 China
Search for more papers by this authorCorresponding Author
Xiang-Hai Cai
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
E-mail: [email protected]Search for more papers by this authorYan Deng
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
These authors contributed to this work equally.
Search for more papers by this authorYang Yu
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
These authors contributed to this work equally.
Search for more papers by this authorBao-Bao Shi
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorMei-Fen Bao
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorSi-Meng Zhao
iHuman Institute, ShanghaiTech University, Shanghai, 200031 China
Search for more papers by this authorCorresponding Author
Xiang-Hai Cai
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
E-mail: [email protected]Search for more papers by this authorMain observation and conclusion
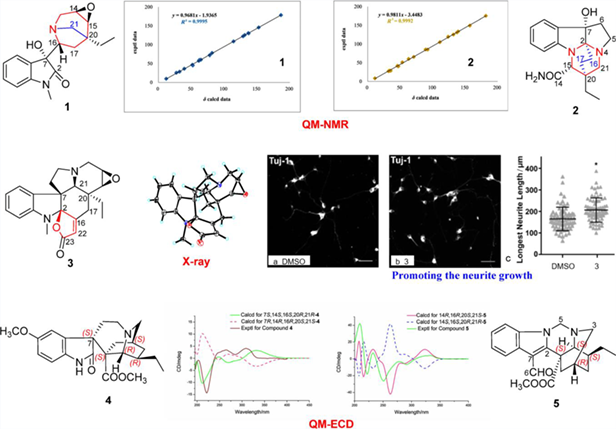
Phytochemical investigations on Tabernaemontana divaricata led to the isolation of seven undescribed monoterpenoid indole alkaloids, taberdicatines A—G (1—7). Taberdicatines A—C might be derived from Aspidosperma-type alkaloid, less skeletal carbons in 1—2 and an additional carbon in 3. Taberdicatines D—E (4—5) were attributed to Iboga alkaloids with enantiomeric skeleton, respectively. Alkaloid 3 could promote the neurite growth of mouse primary cortical neurons at the concentration of 5 μmol/L.
Supporting Information
| Filename | Description |
|---|---|
| cjoc_202000656_sm_suppl.pdfPDF document, 8.8 MB |
Appendix S1. Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Flora Reipublicae Popularis Sinicae, Tomus 63, Delectis Flora Reipublicae Popularis Sinicae Agendae Academiae Sinicae Edita, Science Press, Beijing, 1977.
- 2 Lim, K. H.; Hiraku, O.; Komiyama, K.; Kam, T. S. Jerantinines A-G, cytotoxic Aspidosperma alkaloids from Tabernaemontana corymbosa. J. Nat. Prod. 2008, 9, 1591–1594.
- 3 Zhang, D. B.; Yu, D. G.; Sun, M.; Zhu, X. X.; Yao, X. J.; Zhou, S. Y.; Chen, J. J.; Gao, K. Ervatamines A-I, anti-inflammatory monoterpenoid indole alkaloids with diverse skeletons from Ervatamia hainanensis. J. Nat. Prod. 2015, 6, 1253–1261.
- 4 Cheng, G. G.; Li, D.; Hou, B.; Li, X. N.; Liu, L.; Chen, Y. Y.; Lunga, P. K.; Khan, A.; Liu, Y. P.; Zuo, Z. L.; Luo, X. D. Melokhanines A-J, bioactive monoterpenoid indole alkaloids with diverse skeletons from Melodinus khasianus. J. Nat. Prod. 2016, 9, 2158–2166.
- 5 Zhou, S. Y.; Zhou, T. L.; Qiu, G.; Huan, X.; Miao, Z. H.; Yang, S. P.; Cao, S.; Fan, F.; Cai, Y. S. Three new cytotoxic monoterpenoid bisindole alkaloids from Tabernaemontana bufalina. Planta Med. 2018, 15, 1127–1133.
- 6 Feng, T.; Cai, X. H.; Li, Y.; Wang, Y. Y.; Liu, Y. P.; Luo, X. D. Melohenines A and B, two unprecedented alkaloids from Melodinus henryi. Org. Lett. 2009, 11, 4834–4837.
- 7 Li, P. T.; Leeuwenberg, A. J. M. Flora of China, Vol. 116, Ed.: Middleton, D. J., Science Press, Beijing, 1995.
- 8 Bao, M. F.; Yan, J. M.; Cheng, G. G.; Li, X. Y.; Liu, Y. P.; Li, Y.; Cai, X. H.; Luo, X. D. Cytotoxic indole alkaloids from Tabernaemontana divaricata. J. Nat. Prod. 2013, 76, 1406–1412.
- 9 Chen, H.; Bai, J.; Fang, Z. F.; Yu, S. S.; Ma, S. G.; Xu, S.; Li, Y.; Qu, J.; Ren, J. H.; Li, L.; Si, Y. K.; Chen, X. G. Indole alkaloids and quassinoids from the stems of Brucea mollis. J. Nat. Prod. 2011, 74, 2438–2445.
- 10 Lim, K. H.; Etoh, T.; Hayashi, M.; Komiyama, K.; Kam, T. S. Conolutinine, a hexacyclic indole alkaloid with a novel ring system incorporating a diazaspiro center and fused oxadiazepine-tetrahydrofuran rings. Tetrahedron Lett. 2009, 50, 752–754.
- 11 Lodewyk, M. W.; Siebert, M. R.; Tantillo, D. J. Computational prediction of 1H and 13C chemical shifts: a useful tool for natural product, mechanistic, and synthetic organic chemistry. Chem. Rev. 2012, 112, 1839–1862.
- 12 Kutateladze, A. G.; Holt, T.; Reddy, D. S. Natural products containing the oxetane and related moieties present additional challenges for structure elucidation: A DU8+ computational case study. J. Org. Chem. 2019, 84, 7575–7586.
- 13 Grimblat, N.; Zanardi, M. M.; Sarotti, A. M. Beyond DP4: an improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534.
- 14 Kunesch, N.; Miet, C.; Poisson, J. Hemisynthesis of pachysiphine and 14,15-epoxy-vincamine. Bull. Soc. Chim. Fr. 1982, 9–10, 285–287.
- 15 Wu, X. Z.; Xie, H. Q.; Long, X. H.; Zhang, J. J.; Huang, T.; Hao, X. J.; Zhang, Y. H. Chemical constituents of Catharanthus roseus. Chin. Pharm. J. 2017, 52, 631–636.
- 16 Zhong, X. H.; Bao, M. F.; Zeng, C. X.; Zhang, B. J.; Wu, J.; Zhang, Y.; Cai, X. H. Polycyclic monoterpenoid indole alkaloids from Alstonia rostrata and their reticulate derivation. Phytochem. Lett. 2017, 20, 77–83.
- 17 Beatty, J. W.; Stephenson, C. R. J. Synthesis of (-)-pseudotabersonine, (-)-pseudovincadifformine, and (+)-coronaridine enabled by photoredox catalysis in flow. J. Am. Chem. Soc. 2014, 36, 10270–10273.
- 18 Kono, M.; Harada, S.; Nozaki, T.; Hashimoto, Y.; Murata, S.; Groger, H.; Kuroda, Y.; Yamada, K.; Takasu, K.; Hamada, Y.; Nemoto, T. Org. Lett. 2019, 21, 3750–3754.
- 19 Battersby, A.; Yeowell, D. A. Alkaloids of Calabash curare structure of macusine B. Proc. Chem. Soc. London 1961, 17–19.
- 20 Yu, Y.; Bao, M. F.; Cai, X. H. Discovery of natural co-occurring enantiomers of monoterpenoid indole alkaloids. Chin. J. Chem. 2020, 38, 866–872.




