A Mild, General, Metal-Free Method for Desulfurization of Thiols and Disulfides Induced by Visible-Light
Wenting Qiu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, No. 800 Dongchuan Rd, Shanghai, 200240 China
Search for more papers by this authorShuai Shi
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, No. 800 Dongchuan Rd, Shanghai, 200240 China
Search for more papers by this authorRuining Li
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, No. 800 Dongchuan Rd, Shanghai, 200240 China
Search for more papers by this authorXianfeng Lin
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, No. 800 Dongchuan Rd, Shanghai, 200240 China
Search for more papers by this authorLiangming Rao
Huzhou Research and Industrialization Center for Technology, Chinese Academy of Sciences, 1366 Hongfeng Road, Huzhou, Zhejiang 313000, China, Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences, Shanghai, 200031 China
Search for more papers by this authorCorresponding Author
Zhankui Sun
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, No. 800 Dongchuan Rd, Shanghai, 200240 China
E-mail: [email protected]Search for more papers by this authorWenting Qiu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, No. 800 Dongchuan Rd, Shanghai, 200240 China
Search for more papers by this authorShuai Shi
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, No. 800 Dongchuan Rd, Shanghai, 200240 China
Search for more papers by this authorRuining Li
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, No. 800 Dongchuan Rd, Shanghai, 200240 China
Search for more papers by this authorXianfeng Lin
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, No. 800 Dongchuan Rd, Shanghai, 200240 China
Search for more papers by this authorLiangming Rao
Huzhou Research and Industrialization Center for Technology, Chinese Academy of Sciences, 1366 Hongfeng Road, Huzhou, Zhejiang 313000, China, Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences, Shanghai, 200031 China
Search for more papers by this authorCorresponding Author
Zhankui Sun
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Pharmacy, Shanghai Jiao Tong University, No. 800 Dongchuan Rd, Shanghai, 200240 China
E-mail: [email protected]Search for more papers by this authorMain observation and conclusion
A visible-light-induced metal-free desulfurization method for thiols and disulfides has been explored. This radical desulfurization features mild conditions, robustness, and excellent functionality compatibility. It was successfully applied not only to the desulfurization of small molecules, but also to peptides.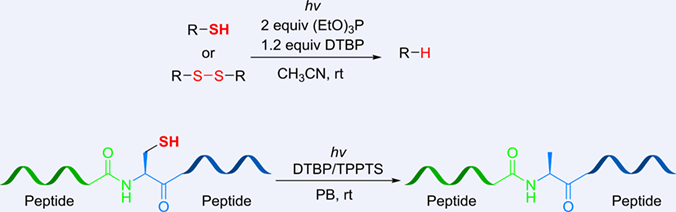
Supporting Information
| Filename | Description |
|---|---|
| cjoc202000607-sup-0001-Supinfo.pdfPDF document, 2.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Magnus, P. D. Recent developments in sulfone chemistry. Tetrahedron 1977, 33, 2019–2045; (b) Eisch, J.; Hallenbeck, L.; Han, K. Hydrodesulfurization of organosulfur heterocycles by metal hydride-nickel (0) complexes: accelerated single-electron transfer in carbon-sulfur bond cleavage. J. Am. Chem. Soc. 1986, 108, 7763–7767; (c) Feng, M. H.; Tang, B. Q.; Liang, S. H.; Jiang, X. F. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216; (d) Zhao, J.; Jiang, X. F. The application of sulfur-containing peptides in drug discovery. Chin. Chem. Lett. 2018, 29, 1079–1087; (e) Wang, N.; Saidhareddy, P.; Jiang, X. F. Construction of sulfur-containing moieties in the total synthesis of natural products. Nat. Prod. Rep. 2020, 37, 246–275.
- 2(a) Eisch, J. J.; Hallenbeck, L. E.; Lucarelli, M. A. Desulphurization and denitrogenation of SRC liquids by transition metals on solid supports. Fuel 1985, 64, 440–442; (b) Schreiner, B. Der Claus-Prozess. Reich an Jahren und bedeutender denn je: Herrn Dr.-Ing. Michael Heisel zum 60. Geburtstag gewidmet. Chem. Unserer Zeit 2008, 42, 378–392; (c) Cook, E.; Peregrine, P. The inventor of the contact process for sulphuric acid. Nature 1926, 117, 419–421.
- 3(a) Mozingo, R.; Wolf, D. E.; Harris, S. A.; Folkers, K. Hydrogenolysis of sulfur compounds by Raney nickel catalyst. J. Am. Chem. Soc. 1943, 65, 1013–1016; (b) Snyder, H.; Cannon, G. W. Carbon—Carbon Cleavage in the Hydrogenolysis by Raney Nickel Catalyst of Ethylenedithiol and its Ethers. J. Am. Chem. Soc. 1944, 66, 155–156; (c) Blicke, F.; Sheets, D. G. Derivatives of Thianaphthene. I1. J. Am. Chem. Soc. 1948, 70, 3768–3770; (d) Hauptmann, H.; Walter, W. F. The Action of Raney Nickel on Organic Sulfur Compounds. Chem. Rev. 1962, 62, 347–404; (e) Yan, L. Z.; Dawson, P. E. Synthesis of peptides and proteins without cysteine residues by native chemical ligation combined with desulfurization. J. Am. Chem. Soc. 2001, 123, 526–533.
- 4(a) Alper, H.; Sibtain, F.; Heveling, J. Phase transfer catalyzed desulfurization reactions. Tetrahedron Lett. 1983, 24, 5329–5332; (b) Alper, H.; Prince, T. L. Äußerst milde Entschwefelung von Thiolen mit Natrium-triethylhydroborat und Übergangsmetallchloriden. Angew. Chem. 1980, 92, 321–321; (c) Alper, H.; Sibtain, F. Desulfurization of benzylic mercaptans by triiron dodecacarbonyl under acidic and biphasic conditions. J. Organomet. Chem. 1985, 285, 225–229.
- 5 Shim, S. C.; Antebi, S.; Alper, H. Desulfurization of mercaptans to hydrocarbons by carbon monoxide and water in the presence of cobalt carbonyl. Tetrahedron Lett. 1985, 26, 1935–1938.
- 6 Wang, Z.; Kuninobu, Y.; Kanai, M. Molybdenum-Mediated Desulfurization of Thiols and Disulfides. Synlett 2014, 25, 1869–1872.
- 7(a) Dawson, P. E.; Muir, T. W.; Clark-Lewis, I.; Kent, S. Synthesis of proteins by native chemical ligation. Science 1994, 266, 776–779; (b) Dawson, P. E.; Kent, S. B. Synthesis of native proteins by chemical ligation. Annu. Rev. Biochem. 2000, 69, 923–960; (c) Ma, J.; Zeng, J.; Wan, Q. Postligation-Desulfurization: A General Approach for Chemical Protein Synthesis. Top. Curr. Chem. 2014, 363, 57–101; (d) Jin, K.; Li, X. C. Advances in Native Chemical Ligation-Desulfurization: A Powerful Strategy for Peptide and Protein Synthesis. Chem.-Eur. J. 2018, 24, 17397–17404; (e) Wang, S. Y.; Thopate, Y. A.; Zhou, Q. Q.; Wang, P. Chemical Protein Synthesis by Native Chemical Ligation and Variations Thereof. Chin. J. Chem. 2019, 37, 1181–1193; (f) Chow, H. Y.; Zhang, Y.; Matheson, E.; Li, X. C. Ligation Technologies for the Synthesis of Cyclic Peptides. Chem. Rev. 2019, 119, 9971–10001; (g) Yin, H.; Zheng, M.; Chen, H.; Wang, S.; Zhou, Q.; Zhang, Q.; Wang, P. Stereoselective and Divergent Construction of β-Thiolated/Selenolated Amino Acids via Photoredox-Catalyzed Asymmetric Giese Reaction. J. Am. Chem. Soc. 2020, 142, 14201–14209.
- 8 Wan, Q.; Danishefsky, S. J. Free-radical-based, specific desulfurization of cysteine: a powerful advance in the synthesis of polypeptides and glycopolypeptides. Angew. Chem. Int. Ed. 2007, 46, 9248–9252.
- 9 Gao, X. F.; Du, J. J.; Liu, Z.; Guo, J. Visible-light-induced specific desulfurization of cysteinyl peptide and glycopeptide in aqueous solution. Org. Lett. 2016, 18, 1166–1169.
- 10 Jin, K.; Li, T.; Chow, H. Y.; Liu, H.; Li, X. P−B Desulfurization: An Enabling Method for Protein Chemical Synthesis and Site-Specific Deuteration. Angew. Chem. Int. Ed. 2017, 56, 14607–14611.
- 11 Shi, S.; Li, R.; Rao, L.; Sun, Z. A mild, general, and metal-free method for site specificdeuteration induced by visible light using D2O as the source of deuterium atoms. Green Chem. 2020, 22, 669.




