Catalytic Benzylation Reactions: From C—H Bond Activation to C—N Bond Activation
Tianxiao Jiang
Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorHongchi Liu
Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorHaocheng Zhang
Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorCorresponding Author
Hanmin Huang
Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
Center for Excellence in Molecular Synthesis of CAS, Hefei, Anhui, 230026 China
E-mail: [email protected]Search for more papers by this authorTianxiao Jiang
Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorHongchi Liu
Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorHaocheng Zhang
Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorCorresponding Author
Hanmin Huang
Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
Center for Excellence in Molecular Synthesis of CAS, Hefei, Anhui, 230026 China
E-mail: [email protected]Search for more papers by this authorAbstract
Transition-metal mediated activation of inert chemical bonds is an ongoing topic in homogeneous catalysis. In view of the abundance and accessibility of alkylarenes and benzylamines, the use of them as benzyl source in catalytic benzylation reactions via benzylic C—H and C—N bond activation is highly desirable. Indeed, compared with the traditional approaches with benzyl halide as the substrates, benzylation reactions via C—H and C—N bond cleavage provide more efficient, atom-economic strategies to access myriads of synthetically important molecules. In this account, our group's efforts on catalytic benzylation reactions via directed C—H activation, nondirected C—H activation and C—N bond activation are summarized.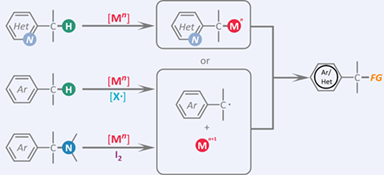
What is the most favorite and original chemistry developed in your research group?
Palladium-catalyzed aminal chemistry.
How do you supervise your students?
Discuss with my students and encourage them to think about where the scientific problem is.
What is the most important personality for scientific research?
Honesty, curiosity and passion.
What are your hobbies?
Reading and playing badminton.
Who influences you mostly in your life?
My parents, who always inspire me to go forward.
What is your favorite journal(s)?
Journals that fairly review the manuscripts and publish inspiring, innovative research results.
References
- 1 Hartwig, J. F. Carbon–heteroatom bond formation catalysed by organometallic complexes. Nature 2008, 455, 314–322.
- 2(a) Shilov, A. E.; Shul'pin, G. B. Activation of C—H bonds by metal complexes. Chem. Rev. 1997, 97, 2879–2932; (b) Chen, X.; Engle, K. M.; Wang, D. H.; Yu, J. Q. Palladium(II)-catalyzed C—H activation/ C—C cross-coupling reactions: versatility and practicality. Angew. Chem. Int. Ed. 2009, 48, 5094–5115; (c) Jun, C. H. Transition metal- catalyzed carbon–carbon bond activation. Chem. Soc. Rev. 2004, 33, 610–618; (d) Ouyang, K.; Hao, W.; Zhang, W. X.; Xi, Z. Transition- metal-catalyzed cleavage of C—N single bonds. Chem. Rev. 2015, 115, 12045–12090; (e) Wang, Q.; Su, Y.; Li, L.; Huang, H. Transition-metal catalysed C–N bond activation. Chem. Soc. Rev. 2016, 45, 1257–1272; (f) Li, B. J.; Yu, D. G.; Sun, C. L.; Shi, Z. J. Activation of “Inert” Alkenyl/Aryl C—O Bond and Its Application in Cross-Coupling Reactions. Chem. - Eur. J. 2011, 17, 1728–1759.
- 3(a) Dexter, D. D.; van der Veen, J. M. Conformations of penicillin G: crystal structure of procaine penicillin G monohydrate and a refinement of the structure of potassium penicillin G. J. Chem. Soc., Perkin Trans. 1 1978, 3, 185–190; (b) Myers, A. G.; Gleason, J. L. A practical synthesis of L-azatyrosine. J. Org. Chem. 1996, 61, 813–815; (c) Kantor, T. G. Ketoprofen: a review of its pharmacologic and clinical properties. Pharmacotherapy 1986, 6, 93–102; (d) Rainsford, K. D. Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology 2009, 17, 275–342; (e) Shi, Q.; Ornstein, P. L.; Briner, K.; Richardson, T. I.; Arnold, M. B.; Backer, R. T.; Buckmaster, J. L.; Canada, E. J.; Doecke, C. W.; Hertel, L. W.; Honigschmidt, N.; Hsiung, H. M.; Husain, S. H.; Kuklish, S. L.; Martinelli, M. J.; Mullaney, J. T.; O'Brien, T. P.; Reinhard, M. R.; Rothhaar, R.; Shah, J.; Wu, Z.; Xie, C.; Zgombick, J. M.; Fisher, M. J. Synthesis and structure–activity relationships of novel dipeptides and reduced dipeptides as ligands for melanocortin subtype-4 receptor. Bioorg. Med. Chem. Lett. 2006, 16, 2341–2346; (f) Christensen, M. K.; Erichsen, K. D.; Trojel-Hansen, C.; Tjørnelund, J.; Nielsen, S. J.; Frydenvang, K.; Johansen, T. N.; Nielsen, B.; Sehested, M.; Jensen, P. B.; Ikaunieks, M.; Zaichenko, A.; Loza, E.; Kalvinsh, I.; Björkling, F. Synthesis and antitumor effect in vitro and in vivo of substituted 1,3-dihydroindole-2-ones. J. Med. Chem. 2010, 53, 7140–7145; (f) Weaver, J. D.; Recio III, A.; Grenning, A. J.; Tunge, J. A. Transition Metal-Catalyzed Decarboxylative Allylation and Benzylation Reactions. Chem. Rev. 2011, 111, 1846–1913.
- 4(a) Shih, H. W.; Vander Wal, M. N.; Grange, R. L.; MacMillan, D. W. Enantioselective α-Benzylation of Aldehydes via Photoredox Organocatalysis. J. Am. Chem. Soc. 2010, 132, 13600–13603; (b) Trost, B. M.; Czabaniuk, L. C. Benzylic Phosphates as Electrophiles in the Palladium-Catalyzed Asymmetric Benzylation of Azlactones. J. Am. Chem. Soc. 2012, 134, 5778–5781; (c) List, B.; Čorić, I.; Grygorenko, O. O.; Kaib, P. S. J.; Komarov, I.; Lee, A.; Leutzsch, M.; Pan, S. C.; Tymtsunik, A. V.; van Gemmeren, M. The Catalytic Asymmetric α-Benzylation of Aldehydes. Angew. Chem. Int. Ed. 2014, 53, 282–285; (d) Lapointe, D.; Fagnou, K. Palladium-catalyzed benzylation of heterocyclic aromatic compounds. Org. Lett. 2009, 11, 4160–4163; (e) Perrone, S.; Bona, F.; Troisi, L. Palladium-catalyzed acylation and/or homo-coupling of aryl- and alkyl-acetylenes. Tetrahedron 2011, 67, 7386–7391.
- 5(a) Trost, B. M.; Czabaniuk, L. C. Benzylic Phosphates as Electrophiles in the Palladium-Catalyzed Asymmetric Benzylation of Azlactones. J. Am. Chem. Soc. 2012, 134, 5778–5781; (b) Trost, B. M.; Czabaniuk, L. C. Palladium-catalyzed asymmetric benzylation of 3-aryl oxindoles. J. Am. Chem. Soc. 2010, 132, 15534–15536; (c) Zhu, Y.; Rawal, V. H. Palladium-catalyzed C3-benzylation of indoles. J. Am. Chem. Soc. 2012, 134, 111–114; (d) Mertins, K.; Iovel, I.; Kischel, J.; Zapf, A.; Beller, M. Transition-Metal-Catalyzed Benzylation of Arenes and Heteroarenes. Angew. Chem. Int. Ed. 2005, 44, 238–242; (e) Schäfer, G.; Bode, J. W. Friedel–Crafts benzylation of activated and deactivated arenes. Angew. Chem. Int. Ed. 2011, 50, 10913–10916; (f) Yang, M. H.; Hunt, J. R.; Sharifi, N.; Altman, R. A. Palladium Catalysis Enables Benzylation of α,α-Difluoroketone Enolates. Angew. Chem. 2016, 128, 9226–9229; Yang, M. H.; Hunt, J. R.; Sharifi, N.; Altman, R. A. Palladium Catalysis Enables Benzylation of α,α-Difluoroketone Enolates. Angew. Chem. Int. Ed. 2016, 55, 9080–9083; (g) Schwarz, K. J.; Yang, C.; Fyfe, J. W.; Snaddon, T. N. Enantioselective α-Benzylation of Acyclic Esters Using π-Extended Electrophiles. Angew. Chem. Int. Ed. 2018, 57, 12102–12105.
- 6(a) Liégault, B.; Renaud, J. L.; Bruneau, C. Activation and Functionalization of Benzylic Derivatives by Palladium Catalysts. Chem. Soc. Rev. 2008, 37, 290–299; (b) Yang, L.; Huang, H. Transition-Metal-Catalyzed Direct Addition of Unactivated C–H Bonds to Polar Unsaturated Bonds. Chem. Rev. 2015, 115, 3468–3517; (c) Vanjari, R.; Singh, K. N. Utilization of Methylarenes as Versatile Building Blocks in Organic Synthesis. Chem. Soc. Rev. 2015, 44, 8062–8096.
- 7(a) Alberico, D.; Scott, M. E.; Lautens, M. Aryl−aryl bond formation by transition-metal-catalyzed direct arylation. Chem. Rev. 2007, 107, 174–238; (b) Lyons, T. W.; Sanford, M. S. Palladium-catalyzed ligand- directed C−H functionalization reactions. Chem. Rev. 2010, 110, 1147–1169.
- 8(a) Ritleng, V.; Sirlin, C.; Pfeffer, M. Ru-, Rh-, and Pd-catalyzed C—C bond formation involving C—H activation and addition on unsaturated substrates: Reactions and mechanistic aspects. Chem. Rev. 2002, 102, 1731–1770; (b) Kakiuchi, F.; Chatani, N. Catalytic methods for C—H bond functionalization: application in organic synthesis. Adv. Synth. Catal. 2003, 345, 1077–1101; (c) Colby, D. A.; Bergman, R. G.; Ellman, J. A. Rhodium-catalyzed C—C bond formation via heteroatom-directed C—H bond activation. Chem. Rev. 2010, 110, 624–655; (d) Wang, R.; Luan, Y.; Ye, M. Transition Metal–Catalyzed Allylic C(sp3)–H Functionalization via η3-Allylmetal Intermediate. Chin. J. Chem. 2019, 37, 720–743.
- 9 Qian, B.; Guo, S.; Shao, J.; Zhu, Q.; Yang, L.; Xia, C.; Huang, H. Palladium-catalyzed benzylic addition of 2-methyl azaarenes to N-sulfonyl aldimines via C−H bond activation. J. Am. Chem. Soc. 2010, 132, 3650–3651.
- 10(a) Cornish, E. J.; Lee, G. E.; Wragg, W. R. 5-Chloro-2-cyclo hexyl-1- oxo-6-sulphamoyl Isoindoline: a New Diuretic. Nature 1963, 197, 1296–1297; (b) Zhu, Q.; Huang, H.; Shi, D.; Shen, Z.; Xia, C. An efficient synthesis of chiral diamines with rigid backbones: Application in enantioselective michael addition of malonates to nitroalkenes. Org. Lett. 2009, 11, 4536–4539.
- 11 Qian, B.; Guo, S.; Xia, C.; Huang, H. Lewis Acid-Catalyzed C—H Functionalization for Synthesis of Isoindolinones and Isoindolines. Adv. Synth. Catal. 2010, 352, 3195–3200.
- 12 Qian, B.; Xie, P.; Xie, Y.; Huang, H. Iron-catalyzed direct alkenylation of 2-substituted azaarenes with N-sulfonyl aldimines via C-H bond activation. Org. Lett. 2011, 13, 2580–2583.
- 13 Qian, B.; Shi, D.; Yang, L.; Huang, H. Lewis Acid-Catalyzed Conjugate Addition of sp3 C—H Bonds to Methylenemalononitriles. Adv. Synth. Catal. 2012, 354, 2146–2150.
- 14 Qian, B.; Yang, L.; Huang, H. Cu-catalyzed direct C–H amination of 2-alkylazaarenes with azodicarboxylates via nucleophilic addition. Tetrahedron Lett. 2013, 54, 711–714.
- 15(a) Rueping, M.; Tolstoluzhsky, N. Copper catalyzed C—H functionalization for direct mannich reactions. Org. Lett. 2011, 13, 1095–1097; (b) Komai, H.; Yoshino, T.; Matsunaga, S.; Kanai, M. Lewis acid catalyzed benzylic C−H bond functionalization of azaarenes: addition to enones. Org. Lett. 2011, 13, 1706–1709; (c) Best, D.; Kujawa, S.; Lam, H. W. Diastereo-and enantioselective Pd (II)-catalyzed additions of 2-alkylazaarenes to N-Boc imines and nitroalkenes. J. Am. Chem. Soc. 2012, 134, 18193–18196; (d) Guan, B. T.; Wang, B.; Nishiura, M.; Hou, Z. Yttrium-Catalyzed Addition of Benzylic C—H Bonds of Alkyl Pyridines to Olefins. Angew. Chem. Int. Ed. 2013, 52, 4418–4421; (e) Jamal, Z.; Teo, Y. C.; Lim, G. S. Direct alkenylation of alkylazaarenes with aldehydes through C(sp3)—H functionalization under catalytic InCl3 activation. Tetrahedron 2016, 72, 2132–2138; (f) Mao, D.; Hong, G.; Wu, S.; Liu, X.; Yu, J.; Wang, L. Lewis-Acid-Catalyzed Benzylic Reactions of 2-Methylazaarenes with Aldehydes. Eur. J. Org. Chem. 2014, 2014, 3009–3019; (g) Graves, V. B.; Shaikh, A. Lewis acid-catalyzed Csp3—H functionalization of methyl azaarenes with α-trifluoromethyl carbonyl compounds. Tetrahedron Lett. 2013, 54, 695–698; (h) Niu, R.; Yang, S.; Xiao, J.; Liang, T.; Li, X. Yb(OTf)3-catalyzed addition of 2-methyl azaarenes to isatins via C—H functionalization. Chin. J. Catal. 2012, 33, 1636–1641.
- 16
Beller, M.; Wu, X. F. Transition Metal Catalyzed Carbonylation Reactions, Springer, Berlin, 2013, pp. 147–166.
10.1007/978-3-642-39016-6 Google Scholar
- 17 Lednicer, D.; Mitscher, L. A. The Organic Chemistry of Drug Synthesis, Vol. 2, John Wiley, New York, 1980, p. 62.
- 18 Li, G.-X.; Huang, H.-M.; Mei, F.-M. A novel synthesis of substituted phenylpyruvic acid by double carbonylation using cobalt pyridine- 2-carboxylate catalyst. Synth. Commun. 2000, 30, 3585–3588.
- 19(a) Blanksby, S. J.; Ellison, G. B. Bond dissociation energies of organic molecules. Acc. Chem. Res. 2003, 36, 255–263; (b) Arnett, E. M.; Amarnath, K.; Harvey, N. G.; Cheng, J. P. Chemical bond-making, bond-breaking, and electron transfer in solution. Science 1990, 247, 423–430.
- 20 Xie, P.; Xie, Y.; Qian, B.; Zhou, H.; Xia, C.; Huang, H. Palladium-catalyzed oxidative carbonylation of benzylic C—H bonds via nondirected C(sp3)—H activation. J. Am. Chem. Soc. 2012, 134, 9902–9905.
- 21 Xie, P.; Xia, C.; Huang, H. Palladium-catalyzed oxidative aminocarbonylation: a new entry to amides via C–H activation. Org. Lett. 2013, 15, 3370–3373.
- 22 Qin, G.; Chen, X.; Yang, L.; Huang, H. Copper-catalyzed α-benzylation of enones via radical-triggered oxidative coupling of two C–H bonds. ACS Catal. 2015, 5, 2882–2885.
- 23 Qin, G.; Wang, Y.; Huang, H. Copper-Catalyzed Dehydrogenative Formal [4+2] and [3+2] Cycloadditions of Methylnaphthalenes and Electron-Deficient Alkenes. Org. Lett. 2017, 19, 6352–6355.
- 24(a) Liu, H.; Laurenczy, G.; Yan, N.; Dyson, P. J. Amide bond formation via C(sp3)—H bond functionalization and CO insertion. Chem. Commun. 2014, 50, 341–343; (b) Powell, D. A.; Fan, H. Copper-Catalyzed Amination of Primary Benzylic C—H Bonds with Primary and Secondary Sulfonamides. J. Org. Chem. 2010, 75, 2726–2729; (c) Chen, C.; Xu, X. H.; Yang, B.; Qing, F. L. Copper-catalyzed direct trifluoromethylthiolation of benzylic C—H bonds via nondirected oxidative C (sp3)–H activation. Org. Lett. 2014, 16, 3372–3375; (d) Zhang, W.; Wang, F.; McCann, S. D.; Wang, D.; Chen, P.; Stahl, S. S.; Liu, G. Enantioselective cyanation of benzylic C—H bonds via copper-catalyzed radical relay. Science 2016, 353, 1014–1018.
- 25(a) Tye, J. W.; Weng, Z.; Johns, A. M.; Incarvito, C. D.; Hartwig, J. F. Copper complexes of anionic nitrogen ligands in the amidation and imidation of aryl halides. J. Am. Chem. Soc. 2008, 130, 9971–9983; (b) Giri, R.; Hartwig, J. F. Cu(I)−amido complexes in the Ullmann Reaction: Reactions of Cu(I)−amido complexes with iodoarenes with and without autocatalysis by CuI. J. Am. Chem. Soc. 2010, 132, 15860–15863.
- 26(a) Liu, Y.; Xie, Y.; Wang, H.; Huang, H. Enantioselective Aminomethylamination of Conjugated Dienes with Aminals Enabled by Chiral Palladium Complex-Catalyzed C—N Bond Activation. J. Am. Chem. Soc. 2016, 138, 4314–4317; (b) Qiao, C.; Chen, A.; Gao, B.; Liu, Y.; Huang, H. Palladium-Catalyzed Cascade Double C—N Bond Activation: A New Strategy for Aminomethylation of 1,3-Dienes with Aminals. Chin. J. Chem. 2018, 36, 929–933.
- 27(a) Maity, P.; Shacklady-McAtee, D. M.; Yap, G. P. A.; Sirianni, E. R.; Watson, M. P. J. Am. Chem. Soc. 2013, 135, 280–285; (b) Basch, C. H.; Cobb, K. M.; Watson, M. P. Org. Lett. 2016, 18, 136–139; (c) Moragas, T.; Gaydou, M.; Martin, R. Angew. Chem. Int. Ed. 2016, 55, 5053–5057; (d) Yi, Y.-Q.-Q.; Yang, W.-C.; Zhai, D.-D.; Zhang, X.-Y.; Li, S.-Q.; Guan, B.-T. Chem. Commun. 2016, 52, 10894–10897; (e) Wang, T.; Yang, S.; Xu, S.; Han, C.; Guo, G.; Zhao, J. RSC Adv. 2017, 7, 15805–15808.
- 28 Anslyn, E. V.; Dougherty, D. A. Modern Physical Organic Chemistry, University Science Books, 2006, pp. 26–33.
- 29 Yada, H.; Tanaka, J.; Nagakura, S. Charge-transfer complexes between iodine and various aliphatic amines. Bull. Chem. Soc. Jpn. 1960, 33, 1660–1667.
- 30 Yu, H.; Gao, B.; Hu, B.; Huang, H. Charge-Transfer Complex Promoted C–N Bond Activation for Ni-Catalyzed Carbonylation. Org. Lett. 2017, 19, 3520–3523.
- 31 Yu, H.; Hu, B.; Huang, H. Nickel-Catalyzed Alkylarylation of Activated Alkenes with Benzyl-amines via C—N Bond Activation. Chem. - Eur. J. 2018, 24, 7114–7117.
- 32 Yu, H.; Hu, B.; Huang, H. Nickel-Catalyzed Benzylation of Aryl Alkenes with Benzylamines via C–N Bond Activation. J. Org. Chem. 2018, 83, 13922–13929.




