Nickel-Catalyzed Dicarbofunctionalization of Alkenes†
Yun-Cheng Luo
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorChang Xu
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Xingang Zhang
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorYun-Cheng Luo
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorChang Xu
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Xingang Zhang
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this author†Dedicated to the 70th Anniversary of Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences.
Abstract
As a straightforward strategy for rapidly increasing molecular complexity, dicarbofunctionalization of alkenes has attracted substantial interests of organic synthesis, medicine chemistry, and materials science. Nickel-catalyzed cascade dicarbofunctionalizations have been flourished in this area recently, and nickel-mediated radical pathways particularly offer new opportunities in conjunctive cross-couplings with alkyl coupling partners. Herein, we give a comprehensive review of nickel-catalyzed dicarbofunctionalization of alkenes through a historical perspective, including intermolecular three-component reactions and intramolecular cascade reactions. Among the pathways discussed in this review, the carbometallation/cross-coupling process and the radical addition/cross-coupling process are the two major pathways for the nickel-catalyzed dicarbofunctionalization of alkenes. The oxidative cyclization and 1,2-metallate shift processes are also selectively discussed. These methods overcome the limitations associated with the reactions using noble metals in the field, providing an efficient and straightforward access to structurally diversified molecules. 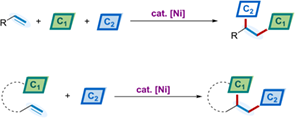
References
- 1 Tang, S.; Liu, K.; Liu, C.; Lei, A. Olefinic C-H Functionalization through Radical Alkenylation. Chem. Soc. Rev. 2015, 44, 1070–1082.
- 2 Coombs, J. R.; Morken, J. P. Catalytic Enantioselective Functionalization of Unactivated Terminal Alkenes. Angew. Chem. Int. Ed. 2016, 55, 2636–2649.
- 3 Sorádová, Z.; Šebesta, R. Enantioselective Cu-Catalyzed Functionalizations of Unactivated Alkenes. ChemCatChem 2016, 8, 2581–2588.
- 4 Dong, Z.; Ren, Z.; Thompson, S. J.; Xu, Y.; Dong, G. Transition-Metal- Catalyzed C-H Alkylation Using Alkenes. Chem. Rev. 2017, 117, 9333–9403.
- 5 Maraswami, M.; Loh, T.-P. Transition-Metal-Catalyzed Alkenyl sp2 C–H Activation: A Short Account. Synthesis 2019, 51, 1049–1062.
- 6 Wang, Z.-X.; Bai, X.-Y.; Li, B.-J. Metal-Catalyzed Substrate-Directed Enantioselective Functionalization of Unactivated Alkenes. Chin. J. Chem. 2019, 37, 1174–1180.
- 7 Chen, J.; Guo, J.; Lu, Z. Recent Advances in Hydrometallation of Alkenes and Alkynes via the First Row Transition Metal Catalysis. Chin. J. Chem. 2018, 36, 1075–1109.
- 8 McDonald, R. I.; Liu, G.; Stahl, S. S. Palladium(II)-Catalyzed Alkene Functionalization via Nucleopalladation: Stereochemical Pathways and Enantioselective Catalytic Applications. Chem. Rev. 2011, 111, 2981–3019.
- 9 Schultz, D. M.; Wolfe, J. P. Recent Developments in Pd-Catalyzed Alkene Aminoarylation Reactions for the Synthesis of Nitrogen Heterocycles. Synthesis 2012, 44, 351–361.
- 10 Ohno, H. Recent Advances in the Construction of Polycyclic Compounds by Palladium-Catalyzed Atom-Economical Cascade Reactions. Asian J. Org. Chem. 2013, 2, 18–28.
- 11 Romero, R. M.; Wöste, T. H.; Muñiz, K. Vicinal Difunctionalization of Alkenes with Iodine(III) Reagents and Catalysts. Chem. Asian J. 2014, 9, 972–983.
- 12 Yin, G.; Mu, X.; Liu, G. Palladium(II)-Catalyzed Oxidative Difunctionalization of Alkenes: Bond Forming at a High-Valent Palladium Center. Acc. Chem. Res. 2016, 49, 2413–2423.
- 13 Lan, X.-W.; Wang, N.-X.; Xing, Y. Recent Advances in Radical Difunctionalization of Simple Alkenes. Eur. J. Org. Chem. 2017, 2017, 5821–5851.
- 14 Wu, Z.; Zhang, W. Recent Advances in Metal-Catalyzed 1,2-Difunctionalization of Conjugated Dienes. Chin. J. Org. Chem. 2017, 37, 2250–2262.
- 15 Koike, T.; Akita, M. New Horizons of Photocatalytic Fluoromethylative Difunctionalization of Alkenes. Chem 2018, 4, 409–437.
- 16 Zhang, J.-S.; Liu, L.; Chen, T.; Han, L.-B. Transition-Metal-Catalyzed Three-Component Difunctionalizations of Alkenes. Chem. Asian J. 2018, 13, 2277–2291.
- 17 Fu, X.; Zhao, W. Progress in Difunctionalization of Alkenes. Chin. J. Org. Chem. 2019, 39, 625–647.
- 18 Kaur, N.; Wu, F.; Alom, N.-E.; Ariyarathna, J. P.; Saluga, S. J.; Li, W. Intermolecular Alkene Difunctionalizations for the Synthesis of Saturated Heterocycles. Org. Biomol. Chem. 2019, 17, 1643–1654.
- 19 Lin, C.; Shen, L. Recent Progress in Transition Metal-Catalyzed Regioselective Functionalization of Unactivated Alkenes/Alkynes Assisted by Bidentate Directing Groups. ChemCatChem 2019, 961–968.
- 20 Lin, J.; Song, R.-J.; Hu, M.; Li, J.-H. Recent Advances in the Intermolecular Oxidative Difunctionalization of Alkenes. Chem. Rec. 2019, 19, 440–451.
- 21 Tian, Y.; Chen, S.; Gu, Q.-S.; Lin, J.-S.; Liu, X.-Y. Amino- and Azidotrifluoromethylation of Alkenes. Tetrahedron Lett. 2018, 59, 203–215.
- 22 Dhungana, R. K.; KC, S.; Basnet, P.; Giri, R. Transition Metal-Catalyzed Dicarbofunctionalization of Unactivated Olefins. Chem. Rec. 2018, 18, 1314–1340.
- 23 Giri, R.; KC, S. Strategies toward Dicarbofunctionalization of Unactivated Olefins by Combined Heck Carbometalation and Cross-Coupling. J. Org. Chem. 2018, 83, 3013–3022.
- 24 Nakao, Y. Nickel/Lewis Acid-Catalyzed Carbocyanation of Unsaturated Compounds. Bull. Chem. Soc. Jpn. 2012, 85, 731–745.
- 25 Derosa, J.; Apolinar, O.; Kang, T.; Tran, V. T.; Engle, K. M. Recent Developments in Nickel-Catalyzed Intermolecular Dicarbofunctionalization of Alkenes. Chem. Sci. 2020, 11, 4287–4296.
- 26 Urkalan, K. B.; Sigman, M. S. Palladium-Catalyzed Oxidative Intermolecular Difunctionalization of Terminal Alkenes with Organostannanes and Molecular Oxygen. Angew. Chem. Int. Ed. 2009, 48, 3146–3149.
- 27 Rosen, B. M.; Quasdorf, K. W.; Wilson, D. A.; Zhang, N.; Resmerita, A.-M.; Garg, N. K.; Percec, V. Nickel-Catalyzed Cross-Couplings Involving Carbon-Oxygen Bonds. Chem. Rev. 2011, 111, 1346–1416.
- 28 Tasker, S. Z.; Standley, E. A.; Jamison, T. F. Recent Advances in Homogeneous Nickel Catalysis. Nature 2014, 509, 299–309.
- 29 Gu, J.; Wang, X.; Xue, W.; Gong, H. Nickel-Catalyzed Reductive Coupling of Alkyl Halides with Other Electrophiles: Concept and Mechanistic Considerations. Org. Chem. Front. 2015, 2, 1411–1421.
- 30 Weix, D. J. Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Acc. Chem. Res. 2015, 48, 1767–1775.
- 31 Tobisu, M.; Chatani, N. Nickel-Catalyzed Cross-Coupling Reactions of Unreactive Phenolic Electrophiles via C-O Bond Activation. Top. Curr. Chem. 2016, 374, 41.
- 32 Lucas, E. L.; Jarvo, E. R. Stereospecific and Stereoconvergent Cross-Couplings between Alkyl Electrophiles. Nat. Rev. Chem. 2017, 1, 0065.
- 33 Guo, L.; Rueping, M. Decarbonylative Cross-Couplings: Nickel Catalyzed Functional Group Interconversion Strategies for the Construction of Complex Organic Molecules. Acc. Chem. Res. 2018, 51, 1185–1195.
- 34 Diccianni, J. B.; Diao, T. Mechanisms of Nickel-Catalyzed Cross- Coupling Reactions. Trends Chem. 2019, 1, 830–844.
- 35 Diccianni, J.; Lin, Q.; Diao, T. Mechanisms of Nickel-Catalyzed Coupling Reactions and Applications in Alkene Functionalization. Acc. Chem. Res. 2020, 53, 906–919.
- 36 Zhu, R.; Buchwald, S. L. Copper-Catalyzed Oxytrifluoromethylation of Unactivated Alkenes. J. Am. Chem. Soc. 2012, 134, 12462–12465.
- 37 Zhu, R.; Buchwald, S. L. Versatile Enantioselective Synthesis of Functionalized Lactones via Copper-Catalyzed Radical Oxyfunctionalization of Alkenes. J. Am. Chem. Soc. 2015, 137, 8069–8077.
- 38 Wang, F.; Chen, P.; Liu, G. Copper-Catalyzed Radical Relay for Asymmetric Radical Transformations. Acc. Chem. Res. 2018, 51, 2036–2046.
- 39 Gu, Q.-S.; Li, Z.-L.; Liu, X.-Y. Copper(I)-Catalyzed Asymmetric Reactions Involving Radicals. Acc. Chem. Res. 2020, 53, 170–181.
- 40 Li, Z.-L.; Fang, G.-C.; Gu, Q.-S.; Liu, X.-Y. Recent Advances in Copper- Catalysed Radical-Involved Asymmetric 1,2-Difunctionalization of Alkenes. Chem. Soc. Rev. 2020, 49, 32–48.
- 41 Montgomery, J. Nickel-Catalyzed Reductive Cyclizations and Couplings. Angew. Chem. Int. Ed. 2004, 43, 3890–3908.
- 42 Liu, Y.; Bandini, M. Nickel Catalyzed Functionalization of Allenes. Chin. J. Chem. 2019, 37, 431–441.
- 43 Ping, Y.; Wang, K.; Pan, Q.; Ding, Z.; Zhou, Z.; Guo, Y.; Kong, W. Ni-Catalyzed Regio- and Enantioselective Domino Reductive Cyclization: One-Pot Synthesis of 2,3-Fused Cyclopentannulated Indolines. ACS Catal. 2019, 9, 7335–7342.
- 44 Chen, J.; Wang, Y.; Ding, Z.; Kong, W. Synthesis of Bridged Tricyclo[5.2.1.01,5]decanes via Nickel-Catalyzed Asymmetric Domino Cyclization of Enynones. Nat. Coummun. 2020, 11, 1882.
- 45 Catellani, M.; Chiusoli, G. P.; Mari, A. Palladium- or Nickel-Catalyzed Sequential Reaction of Organic Bromides, Bicyclo[2.2.1]Hept-2-Ene or Bicyclo[2.2.1]Hepta-2,5-Diene and Alkynes. J. Organomet. Chem. 1984, 275, 129–138.
- 46 Nakao, Y.; Yada, A.; Satoh, J.; Ebata, S.; Oda, S.; Hiyama, T. Arylcyanation of Norbornene and Norbornadiene Catalyzed by Nickel. Chem. Lett. 2006, 35, 790–791.
- 47 Yada, A.; Ebata, S.; Idei, H.; Zhang, D.; Nakao, Y.; Hiyama, T. Nickel/Lewis Acid-Catalyzed Aryl- and Alkenylcyanation of Unsaturated Bonds. Bull. Chem. Soc. Jpn. 2010, 83, 1170–1184.
- 48 Kadam, A. A.; Metz, T. L.; Qian, Y.; Stanley, L. M. Ni-Catalyzed Three-Component Alkene Carboacylation Initiated by Amide C–N Bond Activation. ACS Catal. 2019, 9, 5651–5656.
- 49
Kimura, M.; Matsuo, S.; Shibata, K.; Tamaru, Y. Nickel(0)-Catalyzed Three-Component Connection Reaction of Dimethylzinc, 1,3-Dienes, and Carbonyl Compounds. Angew. Chem. Int. Ed. 1999, 38, 3386–3388.
10.1002/(SICI)1521-3773(19991115)38:22<3386::AID-ANIE3386>3.0.CO;2-W CAS PubMed Web of Science® Google Scholar
- 50 Kojima, K.; Kimura, M.; Tamaru, Y. Nickel-Catalyzed Four-Component Connection of Oraganoaluminium (Organozinc), Isoprene, Aldehydes and Amines: Stereo- and Regioselective Synthesis of Trisubstituted (E)-Homoallylamines. Chem. Commun. 2005, 4717–4719.
- 51 Kimura, M.; Tatsuyama, Y.; Kojima, K.; Tamaru, Y. Alkyne as a Spectator Ligand for the Nickel-Catalyzed Multicomponent Connection Reaction of Diphenylzinc, 1,3-Butadiene, Aldehydes, and Amines. Org. Lett. 2007, 9, 1871–1873.
- 52 Kimura, M.; Ezoe, A.; Mori, M.; Tamaru, Y. Nickel-Catalyzed Addition of Dimethylzinc to Aldehydes Across Alkynes and 1,3-Butadiene: An Efficient Four-Component Connection Reaction. J. Am. Chem. Soc. 2005, 127, 201–209.
- 53 Terao, J.; Oda, A.; Ikumi, A.; Nakamura, A.; Kuniyasu, H.; Kambe, N. Nickel-Catalyzed Dimerization and Carbosilylation of 1,3-Butadienes with Chlorosilanes and Grignard Reagents. Angew. Chem. Int. Ed. 2003, 42, 3412–3414.
- 54 Iwasaki, T.; Fukuoka, A.; Min, X.; Yokoyama, W.; Kuniyasu, H.; Kambe, N. Multicomponent Coupling Reaction of Perfluoroarenes with 1,3-Butadiene and Aryl Grignard Reagents Promoted by an Anionic Ni(II) Complex. Org. Lett. 2016, 18, 4868–4871.
- 55 Iwasaki, T.; Min, X.; Fukuoka, A.; Kuniyasu, H.; Kambe, N. Nickel-Catalyzed Dimerization and Alkylarylation of 1,3-Dienes with Alkyl Fluorides and Aryl Grignard Reagents. Angew. Chem. Int. Ed. 2016, 55, 5550–5554.
- 56 Iwasaki, T.; Fukuoka, A.; Yokoyama, W.; Min, X.; Hisaki, I.; Yang, T.; Ehara, M.; Kuniyasu, H.; Kambe, N. Nickel-Catalyzed Coupling Reaction of Alkyl Halides with Aryl Grignard Reagents in the Presence of 1,3-Butadiene: Mechanistic Studies of Four-Component Coupling and Competing Cross-Coupling Reactions. Chem. Sci. 2018, 9, 2195–2211.
- 57 Terao, J.; Nii, S.; Chowdhury, F. A.; Nakamura, A.; Kambe, N. Nickel-Catalyzed Regioselective Three Component Coupling Reaction of Alkyl Halides, Butadienes, and Ar-M (M=MgX, ZnX). Adv. Synth. Catal. 2004, 346, 905–908.
- 58 Xiao, Y.-L.; Guo, W.-H.; He, G.-Z.; Pan, Q.; Zhang, X. Nickel-Catalyzed Cross-Coupling of Functionalized Difluoromethyl Bromides and Chlorides with Aryl Boronic Acids: A General Method for Difluoroalkylated Arenes. Angew. Chem. Int. Ed. 2014, 53, 9909–9913.
- 59 Feng, Z.; Xiao, Y.-L.; Zhang, X. Transition-Metal (Cu, Pd, Ni)-Catalyzed Difluoroalkylation via Cross-Coupling with Difluoroalkyl Halides. Acc. Chem. Res. 2018, 51, 2264–2278.
- 60 Fu, X.-P.; Xiao, Y.-L.; Zhang, X. Nickel-Catalyzed Difluoromethylation of Arylboronic Acids with Bromodifluoromethane. Chin. J. Chem. 2018, 36, 143–146.
- 61 Gu, J.-W.; Min, Q.-Q.; Yu, L.-C.; Zhang, X. Tandem Difluoroalkylation- Arylation of Enamides Catalyzed by Nickel. Angew. Chem. Int. Ed. 2016, 55, 12270–12274.
- 62 García-Domínguez, A.; Li, Z.; Nevado, C. Nickel-Catalyzed Reductive Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc. 2017, 139, 6835–6838.
- 63 Shrestha, B.; Basnet, P.; Dhungana, R. K.; KC, S.; Thapa, S.; Sears, J. M.; Giri, R. Ni-Catalyzed Regioselective 1,2-Dicarbofunctionalization of Olefins by Intercepting Heck Intermediates as Imine-Stabilized Transient Metallacycles. J. Am. Chem. Soc. 2017, 139, 10653–10656.
- 64 Derosa, J.; Tran, V. T.; Boulous, M. N.; Chen, J. S.; Engle, K. M. Nickel-Catalyzed β,γ-Dicarbofunctionalization of Alkenyl Carbonyl Compounds via Conjunctive Cross-Coupling. J. Am. Chem. Soc. 2017, 139, 10657–10660.
- 65 Li, W.; Boon, J. K.; Zhao, Y. Nickel-Catalyzed Difunctionalization of Allyl Moieties Using Organoboronic Acids and Halides with Divergent Regioselectivities. Chem. Sci. 2018, 9, 600–607.
- 66 Zhao, X.; Tu, H.-Y.; Guo, L.; Zhu, S.; Qing, F.-L.; Chu, L. Intermolecular Selective Carboacylation of Alkenes via Nickel-Catalyzed Reductive Radical Relay. Nat. Coummun. 2018, 9, 3488.
- 67 Yang, Z.-F.; Xu, C.; Zheng, X.; Zhang, X. Nickel-Catalyzed Carbodifunctionalization of N-Vinylamides Enableavavs Access to γ-Amino Acids. Chem. Commun. 2020, 56, 2642–2645.
- 68 Guo, L.; Tu, H.-Y.; Zhu, S.; Chu, L. Selective, Intermolecular Alkylarylation of Alkenes via Photoredox/Nickel Dual Catalysis. Org. Lett. 2019, 21, 4771–4776.
- 69 Tu, H.-Y.; Wang, F.; Huo, L.; Li, Y.; Zhu, S.; Zhao, X.; Li, H.; Qing, F.-L.; Chu, L. Enantioselective Three-Component Fluoroalkylarylation of Unactivated Olefins through Nickel-Catalyzed Cross-Electrophile Coupling. J. Am. Chem. Soc. 2020, 142, 9604–9611.
- 70 Xu, C.; Yang, Z.-F.; An, L.; Zhang, X. Nickel-Catalyzed Difluoroalkylation–Alkylation of Enamides. ACS Catal. 2019, 9, 8224–8229.
- 71 Thapa, S.; Dhungana, R. K.; Magar, R. T.; Shrestha, B.; KC, S.; Giri, R. Ni-Catalysed Regioselective 1,2-Diarylation of Unactivated Olefins by Stabilizing Heck Intermediates as Pyridylsilyl-Coordinated Transient Metallacycles. Chem. Sci. 2018, 9, 904–909.
- 72 Basnet, P.; Dhungana, R. K.; Thapa, S.; Shrestha, B.; KC, S.; Sears, J. M.; Giri, R. Ni-Catalyzed Regioselective β,δ-Diarylation of Unactivated Olefins in Ketimines via Ligand-Enabled Contraction of Transient Nickellacycles: Rapid Access to Remotely Diarylated Ketones. J. Am. Chem. Soc. 2018, 140, 7782–7786.
- 73 Basnet, P.; KC, S.; Dhungana, R. K.; Shrestha, B.; Boyle, T. J.; Giri, R. Synergistic Bimetallic Ni/Ag and Ni/Cu Catalysis for Regioselective γ,δ-Diarylation of Alkenyl Ketimines: Addressing β-H Elimination by in Situ Generation of Cationic Ni(II) Catalysts. J. Am. Chem. Soc. 2018, 140, 15586–15590.
- 74 Derosa, J.; Kleinmans, R.; Tran, V. T.; Karunananda, M. K.; Wisniewski, S. R.; Eastgate, M. D.; Engle, K. M. Nickel-Catalyzed 1,2-Diarylation of Simple Alkenyl Amides. J. Am. Chem. Soc. 2018, 140, 17878–17883.
- 75 Derosa, J.; Kang, T.; Tran, V. T.; Wisniewski, S. R.; Karunananda, M. K.; Jankins, T. C.; Xu, K. L.; Engle, K. M. Nickel-Catalyzed 1,2-Diarylation of Alkenyl Carboxylates: A Gateway to 1,2,3-Trifunctionalized Building Blocks. Angew. Chem. Int. Ed. 2020, 59, 1201–1205.
- 76 Tran, V. T.; Li, Z.-Q.; Gallagher, T. J.; Derosa, J.; Liu, P.; Engle, K. M. Integrating Allyl Electrophiles into Nickel-Catalyzed Conjunctive Cross-Coupling. Angew. Chem. Int. Ed. 2020, 59, 7029–7034.
- 77 Pan, R.; Shi, C.; Zhang, D.; Tian, Y.; Guo, S.; Yao, H.; Lin, A. Nickel-Catalyzed Reductive 1,2-Dialkynylation of Alkenes Bearing an 8-Aminoquinoline Directing Group. Org. Lett. 2019, 21, 8915–8920.
- 78 Yang, T.; Chen, X.; Rao, W.; Koh, M. J. Broadly Applicable Directed Catalytic Reductive Difunctionalization of Alkenyl Carbonyl Compounds. Chem 2020, 6, 738–751.
- 79 Derosa, J.; van der Puyl, V. A.; Tran, V. T.; Liu, M.; Engle, K. M. Directed Nickel-Catalyzed 1,2-Dialkylation of Alkenyl Carbonyl Compounds. Chem. Sci. 2018, 9, 5278–5283.
- 80 Dhungana, R. K.; KC, S.; Basnet, P.; Aryal, V.; Chesley, L. J.; Giri, R. Ni(I)-Catalyzed β,δ-Vinylarylation of γ,δ-Alkenyl α-Cyanocarboxylic Esters via Contraction of Transient Nickellacycles. ACS Catal. 2019, 9, 10887–10893.
- 81 Qin, T.; Cornella, J.; Li, C.; Malins, L. R.; Edwards, J. T.; Kawamura, S.; Maxwell, B. D.; Eastgate, M. D.; Baran, P. S. A General Alkyl-Alkyl Cross-Coupling Enabled by Redox-Active Esters and Alkylzinc Reagents. Science 2016, 352, 801–805.
- 82 KC, S.; Dhungana, R. K.; Shrestha, B.; Thapa, S.; Khanal, N.; Basnet, P.; Lebrun, R. W.; Giri, R. Ni-Catalyzed Regioselective Alkylarylation of Vinylarenes via C(sp3)-C(sp3)/C(sp3)-C(sp2) Bond Formation and Mechanistic Studies. J. Am. Chem. Soc. 2018, 140, 9801–9805.
- 83 KC, S.; Dhungana, R. K.; Aryal, V.; Giri, R. Concise Synthesis of a Potential 5-Lipoxygenase Activating Protein (FLAP) Inhibitor and Its Analogs through Late-Stage Alkene Dicarbofunctionalization. Org. Process Res. Dev. 2019, 23, 1686–1694.
- 84 KC, S.; Dhungana, R. K.; Khanal, N.; Giri, R. Nickel-Catalyzed α-Carbonylalkylarylation of Vinylarenes: Expedient Access to γ,γ-Diarylcarbonyl and Aryltetralone Derivatives. Angew. Chem. Int. Ed. 2020, 59, 8047 –8051
- 85 Chierchia, M.; Xu, P.; Lovinger, G. J.; Morken, J. P. Enantioselective Radical Addition/Cross-Coupling of Organozinc Reagents, Alkyl Iodides, and Alkenyl Boron Reagents. Angew. Chem. Int. Ed. 2019, 58, 14245–14249.
- 86 Lovinger, G. J.; Morken, J. P. Ni-Catalyzed Enantioselective Conjunctive Coupling with C(sp3) Electrophiles: A Radical-Ionic Mechanistic Dichotomy. J. Am. Chem. Soc. 2017, 139, 17293–17296.
- 87 Hidasová, D.; Jahn, U. Intermolecular Formation of Two C-C Bonds across Olefins Enabled by Boron-Based Relay Strategies. Angew. Chem. Int. Ed. 2017, 56, 9656–9658.
- 88 Silvi, M.; Sandford, C.; Aggarwal, V. K. Merging Photoredox with 1,2-Metallate Rearrangements: The Photochemical Alkylation of Vinyl Boronate Complexes. J. Am. Chem. Soc. 2017, 139, 5736–5739.
- 89 Kischkewitz, M.; Okamoto, K.; Mück-Lichtenfeld, C.; Studer, A. Radical-Polar Crossover Reactions of Vinylboron Ate Complexes. Science 2017, 355, 936–938.
- 90 García-Domínguez, A.; Mondal, R.; Nevado, C. Dual Photoredox/ Nickel-Catalyzed Three-Component Carbofunctionalization of Alkenes. Angew. Chem. Int. Ed. 2019, 58, 12286–12290.
- 91 Campbell, M. W.; Compton, J. S.; Kelly, C. B.; Molander, G. A. Three- Component Olefin Dicarbofunctionalization Enabled by Nickel/ Photoredox Dual Catalysis. J. Am. Chem. Soc. 2019, 141, 20069–20078.
- 92 Mega, R. S.; Duong, V. K.; Noble, A.; Aggarwal, V. K. Decarboxylative Conjunctive Cross-coupling of Vinyl Boronic Esters Using Metallaphotoredox Catalysis. Angew. Chem. Int. Ed. 2020, 59, 4375–4379.
- 93 Sun, S.-Z.; Duan, Y.; Mega, R. S.; Somerville, R. J.; Martin, R. Site-Selective 1,2-Dicarbofunctionalization of Vinyl Boronates through Dual Catalysis. Angew. Chem. Int. Ed. 2020, 59, 4370–4374.
- 94 Gao, P.; Chen, L.-A.; Brown, M. K. Nickel-Catalyzed Stereoselective Diarylation of Alkenylarenes. J. Am. Chem. Soc. 2018, 140, 10653–10657.
- 95 Anthony, D.; Lin, Q.; Baudet, J.; Diao, T. Nickel-Catalyzed Asymmetric Reductive Diarylation of Vinylarenes. Angew. Chem. Int. Ed. 2019, 58, 3198–3202.
- 96 Chierchia, M.; Law, C.; Morken, J. P. Nickel-Catalyzed Enantioselective Conjunctive Cross-Coupling of 9-BBN Borates. Angew. Chem. Int. Ed. 2017, 56, 11870–11874.
- 97 Shu, W.; García-Domínguez, A.; Quirós, M. T.; Mondal, R.; Cárdenas, D. J.; Nevado, C. Ni-Catalyzed Reductive Dicarbofunctionalization of Nonactivated Alkenes: Scope and Mechanistic Insights. J. Am. Chem. Soc. 2019, 141, 13812–13821.
- 98
Li, Z.; Wu, D.; Ding, C.; Yin, G. Modular Synthesis of Diarylalkanes by Nickel-Catalyzed 1,1-Diarylation of Unactivated Terminal Alkenes. CCS Chem. 2020, 576–582.
10.31635/ccschem.020.202000183 Google Scholar
- 99 Vaupel, A.; Knochel, P. Stereoselective Synthesis of Substituted Tetrahydrofurans and Butyrolactones by a New Nickel-Catalyzed Carbozincation. Tetrahedron Lett. 1994, 35, 8349–8352.
- 100 Vaupel, A.; Knochel, P. Stereoselective Synthesis of Heterocyclic Zinc Reagents via a Nickel-Catalyzed Radical Cyclization. J. Org. Chem. 1996, 61, 5743–5753.
- 101 Beckwith, A. L. J. Regio-Selectivity and Stereo-Selectivity in Radical Reactions. Tetrahedron 1981, 37, 3073–3100.
- 102 Beckwith, A. L. J.; Lawrence, T.; Serelis, A. K. Stereoselectivity of Ring Closure of Substituted Hex-5-enyl Radicals. J. Chem. Soc., Chem. Commun. 1980, 484–485.
- 103 Beckwith, A. L. J.; Schiesser, C. H. Regio- and Stereo-Selectivity of Alkenyl Radical Ring Closure: A Theoretical Study. Tetrahedron 1985, 41, 3925–3941.
- 104 Stadtmüller, H.; Vaupel, A.; Tucker, C. E.; Stüdemann, T.; Knochel, P. Stereoselective Preparation of Polyfunctional Cyclopentane Derivatives by Radical Nickel- or Palladium-Catalyzed Carbozincations. Chem. Eur. J. 1996, 2, 1204–1220.
- 105 Phapale, V. B.; Buñuel, E.; García-Iglesias, M.; Cárdenas, D. J. Ni-Catalyzed Cascade Formation of C(sp3)-C(sp3) Bonds by Cyclization and Cross-Coupling Reactions of Iodoalkanes with Alkyl Zinc Halides. Angew. Chem. Int. Ed. 2007, 46, 8790–8795.
- 106 Guisán-Ceinos, M.; Soler-Yanes, R.; Collado-Sanz, D.; Phapale, V. B.; Buñuel, E.; Cárdenas, D. J. Ni-Catalyzed Cascade Cyclization-Kumada Alkyl-Alkyl Cross-Coupling. Chem. Eur. J. 2013, 19, 8405–8410.
- 107 KC, S.; Basnet, P.; Thapa, S.; Shrestha, B.; Giri, R. Ni-Catalyzed Regioselective Dicarbofunctionalization of Unactivated Olefins by Tandem Cyclization/Cross-Coupling and Application to the Concise Synthesis of Lignan Natural Products. J. Org. Chem. 2018, 83, 2920–2936.
- 108 Yan, C.-S.; Peng, Y.; Xu, X.-B.; Wang, Y.-W. Nickel-Mediated Inter- and Intramolecular Reductive Cross-Coupling of Unactivated Alkyl Bromides and Aryl Iodides at Room Temperature. Chem. Eur. J. 2012, 18, 6039–6048.
- 109 Peng, Y.; Xu, X.-B.; Xiao, J.; Wang, Y.-W. Nickel-Mediated Stereocontrolled Synthesis of Spiroketals via Tandem Cyclization-Coupling of β-Bromo Ketals and Aryl Iodides. Chem. Commun. 2014, 50, 472–474.
- 110 Peng, Y.; Xiao, J.; Xu, X.-B.; Duan, S. M.; Ren, L.; Shao, Y.-L.; Wang, Y.-W. Stereospecific Synthesis of Tetrahydronaphtho[2,3-b]furans Enabled by a Nickel-Promoted Tandem Reductive Cyclization. Org. Lett. 2016, 18, 5170–5173.
- 111 Xiao, J.; Wang, Y.-W.; Peng, Y. Nickel-Promoted Reductive Cyclization Cascade: A Short Synthesis of a New Aromatic Strigolactone Analogue. Synthesis 2017, 49, 3576–3581.
- 112 Xiao, J.; Cong, X.-W.; Yang, G.-Z.; Wang, Y.-W.; Peng, Y. Stereoselective Synthesis of A Podophyllum Lignan Core by Intramolecular Reductive Nickel-Catalysis. Chem. Commun. 2018, 54, 2040–2043.
- 113 Kuang, Y.; Wang, X.; Anthony, D.; Diao, T. Ni-Catalyzed Two-Component Reductive Dicarbofunctionalization of Alkenes via Radical Cyclization. Chem. Commun. 2018, 54, 2558–2561.
- 114 Wakabayashi, K.; Yorimitsu, H.; Oshima, K. Cobalt-Catalyzed Tandem Radical Cyclization and Cross-Coupling Reaction: Its Application to Benzyl-Substituted Heterocycles. J. Am. Chem. Soc. 2001, 123, 5374–5375.
- 115 You, W.; Brown, M. K. Diarylation of Alkenes by A Cu-Catalyzed Migratory Insertion/Cross-Coupling Cascade. J. Am. Chem. Soc. 2014, 136, 14730–14733.
- 116 Kim, J. G.; Son, Y. H.; Seo, J. W.; Kang, E. J. Iron-Catalyzed Tandem Cyclization and Cross-Coupling Reactions of Iodoalkanes and Aryl Grignard Reagents. Eur. J. Org. Chem. 2015, 2015, 1781–1789.
- 117 You, W.; Brown, M. K. Catalytic Enantioselective Diarylation of Alkenes. J. Am. Chem. Soc. 2015, 137, 14578–14581.
- 118 Thapa, S.; Basnet, P.; Giri, R. Copper-Catalyzed Dicarbofunctionalization of Unactivated Olefins by Tandem Cyclization/Cross-Coupling. J. Am. Chem. Soc. 2017, 139, 5700–5703.
- 119 Jung, M. E.; Piizzi, G. gem-Disubstituent Effect: Theoretical Basis and Synthetic Applications. Chem. Rev. 2005, 105, 1735–1766.
- 120 Lin, Q.; Diao, T. Mechanism of Ni-Catalyzed Reductive 1,2-Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc. 2019, 141, 17937–17948.
- 121 Wang, X.; Ma, G.; Peng, Y.; Pitsch, C. E.; Moll, B. J.; Ly, T. D.; Wang, X.; Gong, H. Ni-Catalyzed Reductive Coupling of Electron-Rich Aryl Iodides with Tertiary Alkyl Halides. J. Am. Chem. Soc. 2018, 140, 14490–14497.
- 122 Huang, D.; Olivieri, D.; Sun, Y.; Zhang, P.; Newhouse, T. R. Nickel-Catalyzed Difunctionalization of Unactivated Alkenes Initiated by Unstabilized Enolates. J. Am. Chem. Soc. 2019, 141, 16249–16254.
- 123 Solé, D.; Cancho, Y.; Llebaria, A.; Moretó, J. M.; Delgado, A. Intramolecular Nitrogen Assistance in the Nickel-Promoted Tandem Cyclization-Capture of Amino-Tethered Vinyl Bromides and Alkenes. J. Am. Chem. Soc. 1994, 116, 12133–12134.
- 124 Solé, D.; Cancho, Y.; Llebaria, A.; Moretó, J. M.; Delgado, A. Nitrogen Assistance in Intramolecular Nickel-Promoted Tandem Cyclization− Quenching Processes. J. Org. Chem. 1996, 61, 5895–5904.
- 125 Watson, M. P.; Jacobsen, E. N. Asymmetric Intramolecular Arylcyanation of Unactivated Olefins via C−CN Bond Activation. J. Am. Chem. Soc. 2008, 130, 12594–12595.
- 126 Nakao, Y.; Ebata, S.; Yada, A.; Hiyama, T.; Ikawa, M.; Ogoshi, S. Intramolecular Arylcyanation of Alkenes Catalyzed by Nickel/AlMe2Cl. J. Am. Chem. Soc. 2008, 130, 12874–12875.
- 127 Hsieh, J.-C.; Ebata, S.; Nakao, Y.; Hiyama, T. Asymmetric Synthesis of Indolines Bearing a Benzylic Quaternary Stereocenter through Intramolecular Arylcyanation of Alkenes. Synlett 2010, 11, 1709–1711.
- 128 Yamada, Y.; Ebata, S.; Hiyama, T.; Nakao, Y. Synthesis of Rhazinilam through Intramolecular Arylcyanation of Alkenes Catalyzed Cooperatively by Nickel/Aluminum. Tetrahedron 2015, 71, 4413–4417.
- 129 Yen, A.; Lautens, M. Nickel-Catalyzed Intramolecular Arylcyanation for the Synthesis of 3,3-Disubstituted Oxindoles. Org. Lett. 2018, 20, 4323–4327.
- 130 Zhang, T.; Luan, Y.-X.; Zheng, S.-J.; Peng, Q.; Ye, M. Chiral Aluminum Complex Controls Enantioselective Nickel-Catalyzed Synthesis of Indenes: C-CN Bond Activation. Angew. Chem. Int. Ed. 2020, 59, 7439–7443.
- 131 Li, Y.; Wang, K.; Ping, Y.; Wang, Y.; Kong, W. Nickel-Catalyzed Domino Heck Cyclization/Suzuki Coupling for the Synthesis of 3,3-Disubstituted Oxindoles. Org. Lett. 2018, 20, 921–924.
- 132 Wang, K.; Ding, Z.; Zhou, Z.; Kong, W. Ni-Catalyzed Enantioselective Reductive Diarylation of Activated Alkenes by Domino Cyclization/ Cross-Coupling. J. Am. Chem. Soc. 2018, 140, 12364–12368.
- 133 Wang, K.; Kong, W. Enantioselective Reductive Diarylation of Alkenes by Ni-Catalyzed Domino Heck Cyclization/Cross Coupling. Synlett 2019, 30, 1008–1014.
- 134 Tian, Z.-X.; Qiao, J.-B.; Xu, G.-L.; Pang, X.; Qi, L.; Ma, W.-Y.; Zhao, Z.-Z.; Duan, J.; Du, Y.-F.; Su, P.; Liu, X.-Y.; Shu, X.-Z. Highly Enantioselective Cross-Electrophile Aryl-Alkenylation of Unactivated Alkenes. J. Am. Chem. Soc. 2019, 141, 7637–7643.
- 135 Li, Y.; Ding, Z.; Lei, A.; Kong, W. Ni-Catalyzed Enantioselective Reductive Aryl-Alkenylation of Alkenes: Application to the Synthesis of (+)-Physovenine and (+)-Physostigmine. Org. Chem. Front. 2019, 6, 3305–3309.
- 136 Ma, T.; Chen, Y.; Li, Y.; Ping, Y.; Kong, W. Nickel-Catalyzed Enantioselective Reductive Aryl Fluoroalkenylation of Alkenes. ACS Catal. 2019, 9, 9127–9133.
- 137 Xu, S.; Wang, K.; Kong, W. Ni-Catalyzed Reductive Arylacylation of Alkenes toward Carbonyl-Containing Oxindoles. Org. Lett. 2019, 21, 7498–7503.
- 138 Jin, Y.; Wang, C. Nickel-Catalyzed Asymmetric Reductive Arylalkylation of Unactivated Alkenes. Angew. Chem. Int. Ed. 2019, 58, 6722–6726.
- 139 Jin, Y.; Wang, C. Ni-Catalysed Reductive Arylalkylation of Unactivated Alkenes. Chem. Sci. 2019, 10, 1780–1785.
- 140 Jin, Y.; Yang, H.; Wang, C. Nickel-Catalyzed Reductive Arylalkylation via a Migratory Insertion/Decarboxylative Cross-Coupling Cascade. Org. Lett. 2019, 21, 7602–7608.
- 141 Jin, Y.; Yang, H.; Wang, C. Nickel-Catalyzed Asymmetric Reductive Arylbenzylation of Unactivated Alkenes. Org. Lett. 2020, 22, 2724–2729.
- 142 Cong, H.; Fu, G. C. Catalytic Enantioselective Cyclization/Cross- Coupling with Alkyl Electrophiles. J. Am. Chem. Soc. 2014, 136, 3788–3791.
- 143 Murakami, M.; Ashida, S. Nickel-Catalysed Intramolecular Alkene Insertion into Cyclobutanones. Chem. Commun. 2006, 4599–4601.
- 144 Liu, L.; Ishida, N.; Murakami, M. Atom- and Step-Economical Pathway to Chiral Benzobicyclo[2.2.2]Octenones through Carbon-Carbon Bond Cleavage. Angew. Chem. Int. Ed. 2012, 51, 2485–2488.
- 145 Walker, J. A., Jr.; Vickerman, K. L.; Humke, J. N.; Stanley, L. M. Ni-Catalyzed Alkene Carboacylation via Amide C-N Bond Activation. J. Am. Chem. Soc. 2017, 139, 10228–10231.
- 146 Zheng, Y.-L.; Newman, S. G. Nickel-Catalyzed Domino Heck-Type Reactions Using Methyl Esters as Cross-Coupling Electrophiles. Angew. Chem. Int. Ed. 2019, 58, 18159–18164.
- 147 Lee, S.-C.; Guo, L.; Rueping, M. Nickel-Catalyzed exo-Selective Hydroacylation/Suzuki Cross-Coupling Reaction. Chem. Commun. 2019, 55, 14984–14987.
- 148 Lan, Y.; Wang, C. Nickel-Catalyzed Enantioselective Reductive Carbo-Acylation of Alkenes. Commun Chem 2020, 3, 45.
- 149 Fan, P.; Lan, Y.; Zhang, C.; Wang, C. Nickel/Photo-Cocatalyzed Asymmetric Acyl-Carbamoylation of Alkenes. J. Am. Chem. Soc. 2020, 142, 2180–2186.
- 150 Weires, N. A.; Slutskyy, Y.; Overman, L. E. Facile Preparation of Spirolactones by an Alkoxycarbonyl Radical Cyclization-Cross- Coupling Cascade. Angew. Chem. Int. Ed. 2019, 58, 8561–8565.




