Recent Advances and Perspectives in Transition Metal-Catalyzed 1,4-Functionalizations of Unactivated 1,3-Enynes for the Synthesis of Allenes
Liang Fu
State Key Laboratory of Organometallic Chemistry, and Shanghai Hongkong Joint Laboratory in Chemical Synthesis, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorSteffen Greßies
State Key Laboratory of Organometallic Chemistry, and Shanghai Hongkong Joint Laboratory in Chemical Synthesis, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorPinhong Chen
State Key Laboratory of Organometallic Chemistry, and Shanghai Hongkong Joint Laboratory in Chemical Synthesis, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Guosheng Liu
State Key Laboratory of Organometallic Chemistry, and Shanghai Hongkong Joint Laboratory in Chemical Synthesis, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorLiang Fu
State Key Laboratory of Organometallic Chemistry, and Shanghai Hongkong Joint Laboratory in Chemical Synthesis, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorSteffen Greßies
State Key Laboratory of Organometallic Chemistry, and Shanghai Hongkong Joint Laboratory in Chemical Synthesis, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorPinhong Chen
State Key Laboratory of Organometallic Chemistry, and Shanghai Hongkong Joint Laboratory in Chemical Synthesis, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Guosheng Liu
State Key Laboratory of Organometallic Chemistry, and Shanghai Hongkong Joint Laboratory in Chemical Synthesis, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorAbstract
Classic methods for the synthesis of allenes usually introduce only one functional group into products. In this review, we highlight the recent advances and perspectives in the synthesis of allenes by transition metal-catalyzed 1,4-functionalization of unactivated 1,3-enynes. 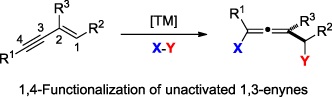
References
- 1(a) Schuster, H. F.; Coppola, G. M. Allenes in Organic Synthesis, Wiley-VCH, New York, 1984; (b) Krause, N.; Hashmi, A. S. K. Modern Allene Chemistry, Wiley-VCH, Weinheim, 2004; (c) Liu, Y.; Bandini, M. Nickel Catalyzed Functionalization of Allenes. Chin. J. Chem. 2019, 37, 431–441.
- 2
Burton, B. S.; von Pechmann, H. Ueber die Einwirkung von Chlorphosphor auf Acetondicarbonsäureäther. Ber. Dtsch. Chem. Ges. 1887, 20, 145–149.
10.1002/cber.18870200136 Google Scholar
- 3 van't Hoff, J. H. La Chimie dans l'Espace, Bazendijk, Rotterdam, 1875.
- 4For reviews on allene synthesis, see: (a) Brummond, K. M.; DeForrest, J. E. Synthesizing Allenes Today (1982—2006). Synthesis 2007, 795–818; (b) Yu, S.; Ma, S. How Easy are the Syntheses of Allenes? Chem. Commun. 2011, 47, 5384–5418; (c) Neff, R. K.; Frantz, D. E. Recent Advances in the Catalytic Syntheses of Allenes: A Critical Assessment. ACS Catal. 2014, 4, 519–528; (d) Ye, J.; Ma, S. Conquering Three- Carbon Axial Chirality of Allenes. Org. Chem. Front. 2014, 1, 1210–1224; (e) Chu, W.-D.; Zhang, Y.; Wang, J. Recent Advances in Catalytic Asymmetric Synthesis of Allenes. Catal. Sci. Technol. 2017, 7, 4570–4579; (f) Huang, X.; Ma, S. Allenation of Terminal Alkynes with Aldehydes and Ketones. Acc. Chem. Res. 2019, 52, 1301–1312.
- 5(a) Jacobs, T. L.; Akawie, R.; Cooper, R. G. Rearrangements Involving 1-Pentyne, 2-Pentyne and 1,2-Pentadiene. J. Am. Chem. Soc. 1951, 73, 1273–1276; (b) Fenandez, I.; Monterde, M. I.; Plumet, J. On the Base-Induced Isomerization of Cyclic Propargylamides to Cyclic Allenamides. Tetrahedron Lett. 2005, 46, 6029–6031; (c) Fotsing, J. R.; Banert, K. First Propargyl Azides Bearing Strong Acceptor Substituents and Their Effective Conversion into Allenyl Azides: Influence of the Electronic Effects of Substituents on the Reactivity of Propargyl Azides. Eur. J. Org. Chem. 2005, 3704–3714; (d) Lepore, S. D.; Khoram, A.; Bromfield, D. C.; Cohn, P.; Jairaj, V.; Silvestri, M. A. Studies on the Manganese-Mediated Isomerization of Alkynyl Carbonyls to Allenyl Carbonyls. J. Org. Chem. 2005, 70, 7443–7446; (e) Brossat, M.; Heck, M.-P.; Mioskowski, C. Bistrimethylsilylpropargylic Ether: A Versatile Ambident Synthon to Access Substituted Allenyne Ethers and α-Substituted Bispropargylic Alcohols. J. Org. Chem. 2007, 72, 5938–5941.
- 6For selected examples on nucleophilic substitutions, see: (a) Rona, P.; Crabbe, P. Novel Synthesis of Substituted Allenes. J. Am. Chem. Soc. 1969, 91, 3289–3292;
(b) Claesson, A.; Olsson, L.-I. Chiral Allenes are Racemised by Organocuprates. J. Chem. Soc., Chem. Commun. 1979, 524–525;
10.1039/c39790000524 Google Scholar(c) Hammond, G. B. Nucleophilic and Electrophilic Substitutions of Difluoropropargyl Bromides. J. Fluorine Chem. 2006, 127, 476–488; (d) Llerena, D.; Buisine, O.; Aubert, C.; Malacria, M. Synthesis of Variously Substituted Allenediynes and Their Cobalt(I)-Mediated [2+2+2] Cycloaddition Reactions. Tetrahedron 1998, 54, 9373–9392; (e) Krause, N.; Hoffmann-Roeder, A. Synthesis of Allenes with Organometallic Reagents. Tetrahedron 2004, 60, 11671–11694; (f) Watanabe, Y.; Yamazaki, T. Facile Preparation of CF3-Containing 1-Bromoallenes. Synlett 2009, 3352–3354; (g) Garcia Ruano, J. L.; Marcos, V.; Aleman, J. Configurational Control of Benzyl Carbanion- Copper Complexes by Sulfinyl Groups: Synthesis of Optically Pure Allenes with Central and Axial Chirality. Angew. Chem. Int. Ed. 2008, 47, 6836–6839; (h) Ito, H.; Sasaki, Y.; Sawamura, M. Copper(I)-Catalyzed Substitution of Propargylic Carbonates with Diboron: Selective Synthesis of Multisubstituted Allenylboronates. J. Am. Chem. Soc. 2008, 130, 15774–15775; (i) Pu, X.; Ready, J. M. Direct and Stereospecific Synthesis of Allenes via Reduction of Propargylic Alcohols with Cp2Zr(H)Cl. J. Am. Chem. Soc. 2008, 130, 10874–10875; (j) Zheng, Y.; Miao, B.; Qin, A.; Xiao, J.; Liu, Q.; Li, G.; Zhang, L.; Zhang, F.; Guo, Y.; Ma, S. Negishi Coupling for Highly Selective Syntheses of Allenes via Ligand Effect and Mechanistic Study via SAESI-MS/MS. Chin. J. Chem. 2019, 37, 1003–1008.
- 7For selected examples on rearrangements, see: (a) Fischer, J.; Kilpert, C.; Klein, U.; Steglich, W. Stereochemistry of [3.3]-Sismatropic Rearrangements in the Oxazole Series. Tetrahedron 1986, 42, 2063–2074; (b) Yoshida, H.; Takahashi, Y.; Kinoshita, H.; Ukishima, S.; Ogata, T.; Matsumoto, K. Preparation of Some Polyfunctional Indenes and Indanones: The Reaction of Mercapto-Substituted Cyclopropenium Salts with Alcohols and Thiols. Bull. Chem. Soc. Jpn. 1991, 64, 3565–3570; (c) Grissom, J. W.; Huang, D. Tandem Eneyne Allene-Radical Cyclization via [3,3] Sigmatropic Rearrangements. J. Org. Chem. 1994, 59, 5114–5116; (d) Aoyagi, S.; Koyanagi, M.; Takahashi, M.; Shimada, K.; Takikawa, Y. Generation of Allenylthioketene S,S-Dioxides through [3,3] Sigmatropic Rearrangement of Alkynyl Propargyl Sulfones. Tetrahedron Lett. 2007, 48, 1915–1918; (e) Tanaka, K.; Okazaki, E.; Shibata, Y. Cationic Rhodium(I)−dppf Complex-Catalyzed Olefin Isomerization/Propargyl Claisen Rearrangement/Carbonyl Migration Cascade. J. Am. Chem. Soc. 2009, 131, 10822–10823; (f) Madelaine, C.; Valerio, V.; Maulide, N. Unexpected Electrophilic Rearrangements of Amides: A Stereoselective Entry to Challenging Substituted Lactones. Angew. Chem. Int. Ed. 2010, 49, 1583–1586; (g) Jiang, X.; Fu, C.; Ma, S. A Concise Synthesis of (-)- and (+)-trans-Whisky Lactones. Eur. J. Org. Chem. 2010, 687–693; (h) Lv, B.; Jiang, X.; Fu, C.; Ma, S. Highly Regio- and Stereoselective Cyclic Iodoetherification of 4,5-Alkadienols. An Efficient Preparation of 2-(1'(Z)-Iodoalkenyl)tetrahydrofurans. J. Org. Chem. 2009, 74, 438–441; (i) Ma, M.; Peng, L.; Li, C.; Zhang, X.; Wang, J. Highly Stereoselective [2,3]-Sigmatropic Rearrangement of Sulfur Ylide Generated through Cu(I) Carbene and Sulfides. J. Am. Chem. Soc. 2005, 127, 15016–15017.
- 8For selected examples on eliminations, see: (a) Yokota, M.; Fuchibe, K.; Ueda, M.; Mayumi, Y.; Ichikawa, J. Facile Synthesis of 1,1-Difluoroallenes via the Difluorovinylidenation of Aldehydes and Ketones. Org. Lett. 2009, 11, 3994–3997; (b) Yamazaki, T.; Yamamoto, T.; Ichihara, R. Preparation of CF3-Containing 1,3-Di- and 1,1,3-Trisubstituted Allenes. J. Org. Chem. 2006, 71, 6251–6253; (c) Maity, P.; Lepore, S. D. Selective One-Pot Synthesis of Allenyl and Alkynyl Esters from β-Ketoesters. J. Org. Chem. 2009, 74, 158–162; (d) Melaimi, M.; Parameswaran, P.; Donnadieu, B.; Frenking, G.; Bertrand, G. Synthesis and Ligand Properties of a Persistent, All-Carbon Four Membered-Ring Allene. Angew. Chem. Int. Ed. 2009, 48, 4792–4795; (e) Lavallo, V.; Dyker, C. A.; Donnadieu, B.; Bertrand, G. Synthesis and Ligand Properties of Stable Five-Membered-Ring Allenes Containing Only Second-Row Elements. Angew. Chem. Int. Ed. 2008, 47, 5411–5414; (f) Tang, M.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.-Q.; Zhang, W.-X.; Wang, A.-X. New Metal-Free One-Pot Synthesis of Substituted Allenes from Enones. Org. Lett. 2008, 10, 5585–5588.
- 9For selected examples on olefinations, see: (a) Bestmann, H. J.; Hartung, H. Reaktionen mit Phosphinalkylenen, XII. Eine neue Synthese von Allen-carbonsäureestern. Chem. Ber. 1966, 99, 1198–1207;
(b) Hamlet, Z.; Barker, W. D. Reaction of Ketene with Stable Phosphorus Ylids. A General Method of Preparation of Terminal Allenes. Synthesis 1970, 543–544;
10.1055/s-1970-21637 Google Scholar(c) Oppolzer, W.; Chapuis, C. Asymmetric Diels-Alder Reaction of a Chiral Allenic Ester: Enantioselective Synthesis of (-)-β-Santalene. Tetrahedron Lett. 1983, 24, 4665–4668; (d) Marshall, J. A.; Wolf, M. A.; Wallace, E. M. Synthetic Routes to Allenic Acids and Esters and Their Stereospecific Conversion to Butenolides. J. Org. Chem. 1997, 62, 367–371; (e) Li, C.-Y.; Sun, X.-L.; Jing, Q.; Tang, Y. Enantioselective Synthesis of Allenic Esters via an Ylide Route. Chem. Commun. 2006, 2980–2982; (f) Xi, Z.; Zhang, W.-X.; Song, Z.; Zheng, W.; Kong, F.; Takahashi, T. Preparation of Vinyl Allenes from 1-Lithio-1,3-dienyl Phosphine Oxides and Aldehydes by the Wittig- Horner Reaction. J. Org. Chem. 2005, 70, 8785–8789; (g) Huang, X.; Xiong, Z.-C. A Novel One-Pot Three-Component Tandem Michael/ Aldol/Horner-Wadsworth-Emmons (HWE) Reaction of Lithium Alkylselenolates with 1-Alkynylphosphine Oxides and Aldehydes: Facile Synthesis of Selenium-Substituted Allenes. Chem. Commun. 2003, 1714–1715; (h) Tsubouchi, A.; Kira, T.; Takeda, T. Peterson Allenation Using (Z)-(1-Lithio-1-alkenyl)trimethylsilanes. Synlett 2006, 2577–2580.
- 10 Skattebøfl, L. Chemistry of gem-Dihalocyclopropanes-VI: A Novel Synthesis of Cyclopentadienes and Fulvenes. Tetrahedron 1967, 23, 1107–1117.
- 11For selected examples, see: (a) Xu, D.; Li, Z.; Ma, S. Efficient Preparation of Highly Optically Active (S)-(-)-2,3-Allenols and (R)-(+)-2,3-Allenyl Acetates by a Clean Novozym-435-Catalyzed Enzymatic Separation of Racemic 2,3-Allenols. Chem. Eur. J. 2002, 8, 5012–5018;
10.1002/1521-3765(20021104)8:21<5012::AID-CHEM5012>3.0.CO;2-2 CAS PubMed Web of Science® Google Scholar(b) Ahmed, M.; Arnauld, T.; Barrett, A. G. M.; Braddock, D. C.; Flack, K.; Procopiou, P. A. Allene Cross-Metathesis: Synthesis of 1,3-Disubstituted Allenes. Org. Lett. 2000, 2, 551–553; (c) Trost, B. M.; Stiles, D. T. Synthesis of Allenamides by Copper-Catalyzed Coupling of Allenyl Halides with Amides, Carbamates, and Ureas. Org. Lett. 2005, 7, 2117–2120; (d) Persson, A. K. Å.; Johnston, E. V.; Baeckvall, J.-E. Copper-Catalyzed N-Allenylation of Allylic Sulfonamides. Org. Lett. 2009, 11, 3814–3817; (e) Zhao, J.; Liu, Y.; Ma, S. Highly Regioselective Synthesis of Trisubstituted Allenes via Lithiation of 1-Aryl-3-alkyl- propadiene, Subsequent Transmetalation, and Pd-Catalyzed Negishi Coupling Reaction. Org. Lett. 2008, 10, 1521–1523; (f) Woerly, E. M.; Cherney, A. H.; Davis, E. K.; Burke, M. D. Stereoretentive Suzuki- Miyaura Coupling of Haloallenes Enables Fully Stereocontrolled Access to (-)-Peridinin. J. Am. Chem. Soc. 2010, 132, 6941–6943; (g) Chen, B.; Lu, Z.; Chai, G.; Fu, C.; Ma, S. An Efficient Double 1,2-Addition Reaction of 2,3-Allenoates with Allyl Magnesium Chloride. J. Org. Chem. 2008, 73, 9486–9489; (h) He, G.; Xue, C.; Fu, C.; Ma, S. An Efficient Synthesis of Allenyl Perfluoroalkyl Ketones from Mono-1,2-Addition–Elimination Reaction of Allenoates with RfMgX. Synlett 2010, 281–285; (i) Deska, J.; del Pozo Ochoa, C.; Baeckvall, J.-E. Chemoenzymatic Dynamic Kinetic Resolution of Axially Chiral Allenes. Chem. Eur. J. 2010, 16, 4447–4451.
- 12(a) Zhang, W.; Xu, H.; Xu, H.; Tang, W. DABCO-Catalyzed 1,4-Bromolactonization of Conjugated Enynes: Highly Stereoselective Formation of a Stereogenic Center and an Axially Chiral Allene. J. Am. Chem. Soc. 2009, 131, 3832–3833; (b) Qian, H.; Yu, X.; Zhang, J.; Sun, J. Organocatalytic Enantioselective Synthesis of 2,3-Allenoates by Intermolecular Addition of Nitroalkanes to Activated Enynes. J. Am. Chem. Soc. 2013, 135, 18020–18023; (c) Poulsen, P. H.; Li, Y.; Lauridsen, V. H.; Jørgensen, D. K. B.; Palazzo, T. A.; Meazza, M.; Jørgensen, K. A. Organocatalytic Formation of Chiral Trisubstituted Allenes and Chiral Furan Derivatives. Angew. Chem. Int. Ed. 2018, 57, 10661–10665.
- 13 Zhao, J.; Liu, Y.; He, Q.; Li, Y.; Ma, S. Experimental and Theoretical Study of Tunable 1,3-Lithium Shift of Propargylic/Allenylic Species, Transmetallation, and Pd-Catalyzed Cross-Coupling Reactions. Chem. Eur. J. 2009, 15, 11361–11372.
- 14 Matsumoto, Y.; Naito, M.; Hayashi, T. Palladlum(0)-Catalyzed Hydroboration of 1-Buten-3-ynes: Preparation of Allenylboranes. Organometallics 1992, 11, 2732–2734.
- 15 Han, J. W.; Tokunaga, N.; Hayashi, T. Palladium-Catalyzed Asymmetric Hydrosilylation of 4-Substituted 1-Buten-3-ynes. Catalytic Asymmetric Synthesis of Axially Chiral Allenylsilanes. J. Am. Chem. Soc. 2001, 123, 12915–12916.
- 16 Lüken, C.; Moberg, C. Silaborations of 1,3-Enynes-Substrate Controlled Allene/1,3-Diene Selectivity. Org. Lett. 2008, 10, 2505–2508.
- 17 Huang, Y.; del Pozo, J.; Torker, S.; Hoveyda, A. H. Enantioselective Synthesis of Trisubstituted Allenyl−B(pin) Compounds by Phosphine− Cu-Catalyzed 1,3-Enyne Hydroboration. Insights Regarding Stereochemical Integrity of Cu−Allenyl Intermediates. J. Am. Chem. Soc. 2018, 140, 2643–2655.
- 18 Gao, D.-W.; Xiao, Y.; Liu, M.; Liu, Z.; Karunananda, M. K.; Chen, J. S.; Engle, K. M. Catalytic, Enantioselective Synthesis of Allenyl Boronates. ACS Catal. 2018, 8, 3650–3654.
- 19(a) Sang, H. L.; Yu, S.; Ge, S. Copper-Catalyzed Asymmetric Hydroboration of 1,3-Enynes with Pinacolborane to Access Chiral Allenylboronates. Org. Chem. Front. 2018, 5, 1284–1287; (b) Yu, S.; Sang, H. L.; Zhang, S.-Q.; Hong, X.; Ge, S. Catalytic Asymmetric Synthesis of Chiral Trisubstituted Heteroaromatic Allenes from 1,3-Enynes. Commun. Chem. 2018, 1, 64.
- 20 Mori, Y.; Onodera, G.; Kimura, M. Ni-Catalyzed Three-component Coupling Reaction of Conjugated Enyne, Carbonyls, and Dimethylzinc to Construct Allenyl Alcohols. Chem. Lett. 2014, 43, 97–99.
- 21 Adamson, N. J.; Jeddi, H.; Malcolmson, S. J. Preparation of Chiral Allenes through Pd-Catalyzed Intermolecular Hydroamination of Conjugated Enynes: Enantioselective Synthesis Enabled by Catalyst Design. J. Am. Chem. Soc. 2019, 141, 8574–8583.
- 22(a) Zhang, Y.; Yu, B.; Gao, B.; Zhang, T.; Huang, H. Triple-Bond Insertion Triggers Highly Regioselective 1,4-Aminomethylamination of 1,3-Enynes with Aminals Enabled by Pd-Catalyzed C−N Bond Activation. Org. Lett. 2019, 21, 535–539; (b) Xie, Y.; Hu, J.; Wang, Y.; Xia, C.; Huang, H. Palladium-Catalyzed Vinylation of Aminals with Simple Alkenes: A New Strategy To Construct Allylamines. J. Am. Chem. Soc. 2012, 134, 20613–20616.
- 23(a) Melikyan, G. G. Propargyl Radical Chemistry: Renaissance Instigated by Metal Coordination. Acc. Chem. Res. 2015, 48, 1065–1079; (b) Kochi, J. K.; Krusic, P. J. Electron Spin Resonance of Free Radicals from Acetylenes and Allenes. J. Am. Chem. Soc. 1970, 92, 4110–4114; (c) Honjou, H.; Yoshimine, M.; Pacansky, J. Theoretical Studies on the Ground State and Low-Lying Doublet Excited States of the Propargyl Radical. J. Phys. Chem. 1987, 91, 4455–4459; (d) Jochnowitz, E. B.; Zhang, X.; Nimlos, M. R.; Varner, M. E.; Stanton, J. F.; Ellison, G. B. Propargyl Radical: Ab Initio Anharmonic Modes and the Polarized Infrared Absorption Spectra of Matrix-Isolated HCCCH2. J. Phys. Chem. A 2005, 109, 3812–3821; (e) Maury, J.; Jammi, S.; Vibert, F.; Marque, S. R. A.; Siri, D.; Feray, L.; Bertrand, M. EPR Investigation of Zinc/Iodine Exchange between Propargyl Iodides and Diethylzinc: Detection of Propargyl Radical by Spin Trapping. J. Org. Chem. 2012, 77, 9081–9086.
- 24 Wang, F.; Wang, D.; Zhou, Y.; Liang, L.; Lu, R.; Chen, P.; Lin, Z.; Liu, G. Divergent Synthesis of CF3-Substituted Allenyl Nitriles by Ligand- Controlled Radical 1,2- and 1,4-Addition to 1,3-Enynes. Angew. Chem. Int. Ed. 2018, 57, 7140–7145.
- 25 Zhu, X.; Deng, W.; Chiou, M.-F.; Ye, C.; Jian, W.; Zeng, Y.; Jiao, Y.; Ge, L.; Li, Y.; Zhang, X.; Bao, H. Copper-Catalyzed Radical 1,4-Difunctionalization of 1,3-Enynes with Alkyl Diacyl Peroxides and N-Fluorobenzenesulfonimide. J. Am. Chem. Soc. 2019, 141, 548–559.
- 26 Ye, C.; Li, Y.; Zhu, X.; Hu, S.; Yuan, D.; Bao, H. Copper-Catalyzed 1,4-Alkylarylation of 1,3-Enynes with Masked Alkyl Electrophiles. Chem. Sci. 2019, 10, 3632–3636.
- 27 Terao, J.; Bando, F.; Kambe, N. Ni-Catalyzed Regioselective Three- Component Coupling of Alkyl Halides, Arylalkynes, or Enynes with R-M (M = MgX', ZnX'). Chem. Commun. 2009, 7336–7338.
- 28 Zhang, K.-F.; Bian, K.-J.; Li, C.; Sheng, J.; Li, Y.; Wang, X.-S. Nickel-Catalyzed Carbofluoroalkylation of 1,3-Enynes to Access Structurally Diverse Fluoroalkylated Allenes. Angew. Chem. Int. Ed. 2019, 58, 5069–5074.




