Metal Complexes in Cancer Treatment: Journey So Far
Ankit Kumar Singh
Department of Pharmaceutical Sciences and Natural Products, Central University of Punjab, Ghudda, Bathinda, 151401 India
Equal contribution: both are treated as the first author.
Search for more papers by this authorAdarsh Kumar
Department of Pharmaceutical Sciences and Natural Products, Central University of Punjab, Ghudda, Bathinda, 151401 India
Equal contribution: both are treated as the first author.
Search for more papers by this authorHarshwardhan Singh
Department of Pharmaceutical Sciences and Natural Products, Central University of Punjab, Ghudda, Bathinda, 151401 India
Search for more papers by this authorPankaj Sonawane
Department of Pharmaceutical Sciences and Natural Products, Central University of Punjab, Ghudda, Bathinda, 151401 India
Search for more papers by this authorPrateek Pathak
Laboratory of Computational Modeling of Drugs, Higher Medical and Biological School, South Ural State University, Chelyabinsk, 454008 Russia
Search for more papers by this authorMaria Grishina
Laboratory of Computational Modeling of Drugs, Higher Medical and Biological School, South Ural State University, Chelyabinsk, 454008 Russia
Search for more papers by this authorJagat Pal Yadav
Bioorganic and Medicinal Chemistry Research Laboratory, Department of Pharmaceutical Sciences, Sam Higginbottom University of Agriculture, Technology and Sciences, Prayagraj, 211007 India
Pharmacology research Laboratory, Faculty of Pharmaceutical Sciences, Rama University, Kanpur, 209217 India
Search for more papers by this authorAmita Verma
Bioorganic and Medicinal Chemistry Research Laboratory, Department of Pharmaceutical Sciences, Sam Higginbottom University of Agriculture, Technology and Sciences, Prayagraj, 211007 India
Search for more papers by this authorCorresponding Author
Pradeep Kumar
Department of Pharmaceutical Sciences and Natural Products, Central University of Punjab, Ghudda, Bathinda, 151401 India
Search for more papers by this authorAnkit Kumar Singh
Department of Pharmaceutical Sciences and Natural Products, Central University of Punjab, Ghudda, Bathinda, 151401 India
Equal contribution: both are treated as the first author.
Search for more papers by this authorAdarsh Kumar
Department of Pharmaceutical Sciences and Natural Products, Central University of Punjab, Ghudda, Bathinda, 151401 India
Equal contribution: both are treated as the first author.
Search for more papers by this authorHarshwardhan Singh
Department of Pharmaceutical Sciences and Natural Products, Central University of Punjab, Ghudda, Bathinda, 151401 India
Search for more papers by this authorPankaj Sonawane
Department of Pharmaceutical Sciences and Natural Products, Central University of Punjab, Ghudda, Bathinda, 151401 India
Search for more papers by this authorPrateek Pathak
Laboratory of Computational Modeling of Drugs, Higher Medical and Biological School, South Ural State University, Chelyabinsk, 454008 Russia
Search for more papers by this authorMaria Grishina
Laboratory of Computational Modeling of Drugs, Higher Medical and Biological School, South Ural State University, Chelyabinsk, 454008 Russia
Search for more papers by this authorJagat Pal Yadav
Bioorganic and Medicinal Chemistry Research Laboratory, Department of Pharmaceutical Sciences, Sam Higginbottom University of Agriculture, Technology and Sciences, Prayagraj, 211007 India
Pharmacology research Laboratory, Faculty of Pharmaceutical Sciences, Rama University, Kanpur, 209217 India
Search for more papers by this authorAmita Verma
Bioorganic and Medicinal Chemistry Research Laboratory, Department of Pharmaceutical Sciences, Sam Higginbottom University of Agriculture, Technology and Sciences, Prayagraj, 211007 India
Search for more papers by this authorCorresponding Author
Pradeep Kumar
Department of Pharmaceutical Sciences and Natural Products, Central University of Punjab, Ghudda, Bathinda, 151401 India
Search for more papers by this authorAbstract
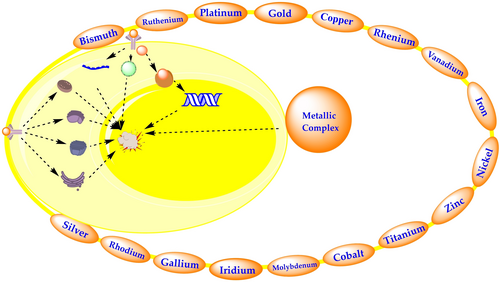
Metal complexes in cancer therapy have attracted much interest mainly because metals exhibit unique characteristics, such as redox activity, metal-ligand interaction, structure and bonding, Lewis acid properties etc. In 1965, Barnett Rosenberg serendipitously discovered the metal-based compound cisplatin, an outstanding breakthrough in the history of metal-based anticancer complexes and led to a new area of anticancer drug discovery. Many metal-based compounds have been studied for their potential anticancer properties. Some of these compounds have FDA approval for clinical use, while others are now undergoing clinical trials for cancer therapy and detection. In the present study, we have highlighted the primary mode of action of metallic complexes and all FDA-approved/under clinical trial drugs with reference to cancer treatment. This review also focuses on recent progress on metal-based complexes such as platinum, ruthenium, iron, etc. with potential anticancer activities.
Graphical Abstract
Conflict of interest
The authors declare no conflict of interest.
References
- 1K. Kasemodel, K. Roberts, ‘In Metal-Based Chemotherapy Drugs’, Proc. Okla. Acad. Sci. 2019, 99, 106–113.
- 2G. Shumi, T. Desalegn, T. B. Demissie, V. P. Ramachandran, R. Eswaramoorthy, ‘Metal Complexes in Target-Specific Anticancer Therapy: Recent Trends and Challenges’, J. Chem. 2022, 2022, 1–19.
- 3N. Farrell, ‘Metal complexes as drugs and chemotherapeutic agents’, Compr. Coord. Chem. II 2003, 9, 809–840.
- 4J. Karges, R. W. Stokes, S. M. Cohen, ‘Metal complexes for therapeutic applications’, Trends Chem. 2021, 3, 523–534.
- 5B. Desoize, ‘Metals and metal compounds in cancer treatment’, Anticancer Res. 2004, 24, 1529–1544.
- 6P. V. Simpson, N. M. Desai, I. Casari, M. Massi, M. Falasca, ‘Metal-based antitumor compounds: beyond cisplatin’, Future Med. Chem. 2019, 11, 119–135.
- 7K. J. Franz, N. Metzler-Nolte, ‘Introduction: metals in medicine’, Chem. Rev. 2019, 119, 727–729.
- 8N. P. Barry, P. J. Sadler, ‘Challenges for metals in medicine: how nanotechnology may help to shape the future’, ACS Nano 2013, 7, 5654–5659.
- 9U. Ndagi, N. Mhlongo, M. E. Soliman, ‘Metal complexes in cancer therapy – an update from drug design perspective’, Drug Des. Dev. Ther. 2017, 11, 599.
- 10R. K. Sodhi, S. Paul, ‘Metal complexes in medicine an overview and update from drug design perspective’, Cancer Ther. Oncol. Int. J. 2019, 14, 25–32.
- 11D. Chen, V. Milacic, M. Frezza, Q. P. Dou, ‘Metal complexes, their cellular targets and potential for cancer therapy’, Curr. Pharm. Des. 2009, 15, 777–791.
- 12M. Frezza, S. Hindo, D. Chen, A. Davenport, S. Schmitt, D. Tomco, Q. Ping Dou, ‘Novel metals and metal complexes as platforms for cancer therapy’, Curr. Pharm. Des. 2010, 16, 1813–1825.
- 13E. J. Anthony, E. M. Bolitho, H. E. Bridgewater, O. W. Carter, J. M. Donnelly, C. Imberti, E. C. Lant, F. Lermyte, R. J. Needham, M. Palau, ‘Metallodrugs are unique: Opportunities and challenges of discovery and development’, Chem. Sci. 2020, 11, 12888–12917.
- 14E. Boros, P. J. Dyson, G. Gasser, ‘Classification of metal-based drugs according to their mechanisms of action’, Chem 2020, 6, 41–60.
- 15E. Alberti, M. Zampakou, D. Donghi, ‘Covalent and non-covalent binding of metal complexes to RNA’, J. Inorg. Biochem. 2016, 163, 278–291.
- 16P. C. Bruijnincx, P. J. Sadler, ‘New trends for metal complexes with anticancer activity’, Curr. Opin. Chem. Biol. 2008, 12, 197–206.
- 17K. Krzywoszyńska, D. Witkowska, J. Świątek-Kozłowska, A. Szebesczyk, H. Kozłowski, ‘General aspects of metal ions as signaling agents in health and disease’, Biomol. Eng. 2020, 10, 1417.
- 18Y. Liu, Y. Wang, S. Song, H. Zhang, ‘Cancer therapeutic strategies based on metal ions’, Chem. Sci. 2021, 12, 12234–12247.
- 19C. M. Che, F. M. Siu, ‘Metal complexes in medicine with a focus on enzyme inhibition’, Curr. Opin. Chem. Biol. 2010, 14, 255–261.
- 20D. Griffith, J. J. Parker, C. ’Marmion, ‘Enzyme inhibition as a key target for the development of novel metal-based anticancer therapeutics’, Anti-Cancer Agents Med. Chem. 2010, 10, 354–370.
- 21P. Pedrosa, A. Carvalho, P. V. Baptista, A. R. Fernandes, ‘Inorganic Coordination Chemistry: Where We Stand in Cancer Treatment?’, Front. Inorg. Coord. Chem. 2018.
- 22P. Zhang, P. J. Sadler, ‘Redox-active metal complexes for anticancer therapy’, Eur. J. Inorg. Chem. 2017, 2017, 1541–1548.
- 23K. Jomova, S. Baros, M. Valko, ‘Redox active metal-induced oxidative stress in biological systems’, Transition Met. Chem. 2012, 37, 127–134.
- 24I. Romero-Canelon, P. J. Sadler, ‘Next-generation metal anticancer complexes: multitargeting via redox modulation’, Inorg. Chem. 2013, 52, 12276–12291.
- 25A. Gaeta, R. C. Hider, ‘The crucial role of metal ions in neurodegeneration: the basis for a promising therapeutic strategy’, Br. J. Pharmacol. 2005, 146, 1041–1059.
- 26Z. Fan, J. Huang, H. Huang, S. Banerjee, ‘Metal-Based Catalytic Drug Development for Next-Generation Cancer Therapy’, ChemMedChem 2021, 16, 2480–2486.
- 27K. D. Mjos, C. Orvig, ‘Metallodrugs in medicinal inorganic chemistry’, Chem. Rev. 2014, 114, 4540–4563.
- 28N. J. Farrer, L. Salassa, P. J. Sadler, ‘Photoactivated chemotherapy (PACT): the potential of excited-state d-block metals in medicine’, Dalton Trans. 2009, 48, 10690–10701.
- 29S. A. McFarland, A. Mandel, R. Dumoulin-White, G. Gasser, ‘Metal-based photosensitizers for photodynamic therapy: the future of multimodal oncology’, Curr. Opin. Chem. Biol. 2020, 56, 23–27.
- 30G. Sgouros, L. Bodei, M. R. McDevitt, J. R. Nedrow, ‘Radiopharmaceutical therapy in cancer: clinical advances and challenges’, Nat. Rev. Drug Discovery 2020, 19, 589–608.
- 31C. F. Ramogida, C. Orvig, ‘Tumor targeting with radiometals for diagnosis and therapy’, Chem. Commun. 2013, 49, 4720–4739.
- 32C. S. Cutler, H. M. Hennkens, N. Sisay, S. Huclier-Markai, S. S. Jurisson, ‘Radiometals for combined imaging and therapy’, Chem. Rev. 2013, 113, 858–883.
- 33M. Callari, J. R. Aldrich-Wright, P. L. de Souza, M. H. Stenzel, ‘Polymers with platinum drugs and other macromolecular metal complexes for cancer treatment’, Prog. Polym. Sci. 2014, 39, 1614–1643.
- 34S. P. Fricker, ‘Metal based drugs: from serendipity to design’, Dalton Trans. 2007, 43, 4903–4917.
- 35A. L. Lainé, C. Passirani, ‘Novel metal-based anticancer drugs: a new challenge in drug delivery’, Curr. Opin. Pharmacol. 2012, 12, 420–426.
- 36M. Kanlayavattanakul, K. Fungpaisalpong, M. Pumcharoen, N. Lourith, ‘In preparation and efficacy assessment of malva nut polysaccharide for skin hydrating products’, Ann. Pharm. Fr. 2017, 75, 436–445.
- 37S. Dasari, P. B. Tchounwou, ‘Cisplatin in cancer therapy: molecular mechanisms of action’, Eur. J. Pharmacol. 2014, 740, 364–378.
- 38G. F. D. Sousa, S. R. Wlodarczyk, G. Monteiro, ‘Carboplatin: molecular mechanisms of action associated with chemoresistance’, Braz. J. Pharm. Sci. 2014, 50, 693–701.
- 39R. Hellweg, A. Mooneyham, Z. Chang, M. Shetty, E. Emmings, Y. Iizuka, C. Clark, T. Starr, J. H. Abrahante, F. Schütz, ‘RNA sequencing of carboplatin-and paclitaxel-resistant endometrial cancer cells reveals new stratification markers and molecular targets for cancer treatment’, Horm. Cancer 2018, 9, 326–337.
- 40D. Simpson, C. Dunn, M. Curran, K. L. Goa, ‘Oxaliplatin’, Drugs 2003, 63, 2127–2156.
- 41J. L. Misset, H. Bleiberg, W. Sutherland, M. Bekradda, E. Cvitkovic, ‘Oxaliplatin clinical activity: a review’, Crit. Rev. Oncol. Hematol. 2000, 35, 75–93.
- 42T. Kunimatsu, J. Shimakura, M. Hanada, ‘Development of miriplatin, a novel antitumor platinum for hepatocellular carcinoma’, Sumitomo Kagaku RandD Rep. 2011, 1, 11–12.
- 43M. J. McKeage, ‘Lobaplatin: a new antitumor platinum drug’, Expert Opin. Invest. Drugs 2001, 10, 119–128.
- 44J. He, H. Zhang, ‘The antitumor effect of lobaplatin against Ishikawa endometrial cancer cells In Vitro and In Vivo’, Biomed. Pharmacother. 2019, 114, 108762.
- 45X. Huang, H. Zhou, R. Jiao, H. Liu, C. Qin, L. Xu, Y. Chen, ‘Supramolecular chemotherapy: Host-guest complexes of Heptaplatin-Cucurbit[7] uril toward colorectal normal and tumor cells’, Langmuir. 2021, 37, 5475–5482.
- 46C. Deng, N. Zhang, S. Jiang, H. Zhang, J. A. Ma, W. Zou, X. Liu, C. Hu, T. Hou, ‘Nedaplatin-based chemotherapy or cisplatin-based chemotherapy combined with intensity-modulated radiotherapy achieve similar efficacy for stage II–IVa nasopharyngeal carcinoma patients’, Sci. Rep. 2022, 12, 1–10.
- 47M. E. Alberto, M. F. A. Lucas, M. Pavelka, N. Russo, ‘The second-generation anticancer drug nedaplatin: a theoretical investigation on the hydrolysis mechanism’, J. Phys. Chem. 2009, 113, 14473–14479.
- 48H. Zhang, R. Chen, X. Wang, H. Zhang, X. Zhu, J. Chen, ‘Lobaplatin-induced apoptosis requires p53-mediated p38MAPK activation through ROS generation in non-small-cell lung cancer’, Front. Oncol. 2019, 9, 538.
- 49A. Noweski, A. Roosen, S. Lebdai, E. Barret, M. Emberton, F. Benzaghou, M. Apfelbeck, B. Gaillac, C. Stief, C. Gratzke, ‘Medium-term follow-up of vascular-targeted photodynamic therapy of localized prostate cancer using TOOKAD soluble WST-11 (Phase II Trials)’, Eur. Urol. Focus 2019, 5, 1022–1028.
- 50B. Habermeyer, R. Guilard, ‘Some activities of PorphyChem illustrated by the applications of porphyrinoids in PDT, PIT and PDI’, Photochem. Photobiol. Sci. 2018, 17, 1675–1690.
- 51G. Obaid, ‘Targeted nanoparticle platforms for selective photodynamic therapy of cancer’, University of East Anglia, 2013.
- 52Y. Zhang, ‘Low Temperature Block Copolymer Processing for Biomedical Applications’, State University of New York at Buffalo, 2016.
- 53W. Huang, Y. Zeng, ‘A candidate for lung cancer treatment: arsenic trioxide’, Clin. Transl. Oncol. 2019, 21, 1115–1126.
- 54A. Kopacz-Bednarska, T. Król, ‘Selected platinum complexes in standard and modern anticancer therapies’, B. Pol. Acad. Sci. Tech. 2022, 7, 106–115.
- 55J. X. Ong, H. V. Le, V. E. Y. Lee, W. H. Ang, ‘A Cisplatin-Selective Fluorescent Probe for Real-Time Monitoring of Mitochondrial Platinum Accumulation in Living Cells’, Angew. Chem. 2021, 133, 9350–9355.
10.1002/ange.202010951 Google Scholar
- 56S. Su, Y. Chen, P. Zhang, R. Ma, W. Zhang, J. Liu, T. Li, H. Niu, Y. Cao, B. Hu, ‘The role of Platinum (IV)-Based antitumor drugs and the anticancer immune response in medicinal inorganic chemistry. A systematic review from 2017 to 2022’, Eur. J. Med. Chem. 2022, 12, 114680.
- 57A. D. Aputen, M. G. Elias, J. Gilbert, J. A. Sakoff, C. P. Gordon, K. F. Scott, J. R. Aldrich-Wright, ‘Potent Chlorambucil-Platinum (IV) Prodrugs’, Int. J. Mol. Sci. 2022, 23, 10471.
- 58A. Bhargava, U. N. Vaishampayan, ‘Satraplatin: leading the new generation of oral platinum agents’, Expert Opin. Invest. Drugs 2009, 18, 1787–1797.
- 59S. Q. Mi, C. Shu, C. Yang, X. Gao, X. Zhang, C. Bao, X. Zhang, J. Niu, ‘Current status for oral platinum (IV) anticancer drug development’, Int. J. Med. Phys. Clin. Eng. Radiat Onco. 2018, 7, 231.
10.4236/ijmpcero.2018.72020 Google Scholar
- 60T. C. Johnstone, K. Suntharalingam, S. J. Lippard, ‘The next generation of platinum drugs: targeted Pt (II) agents, nanoparticle delivery, and Pt (IV) prodrugs’, Chem. Rev. 2016, 116, 3436–3486.
- 61S. Dhar, S. J. Lippard, ‘Mitaplatin, a potent fusion of cisplatin and the orphan drug dichloroacetate’, Proc. Nat. Acad. Sci. 2009, 106, 22199–22204.
- 62N. P. Barry, P. J. Sadler, ‘Exploration of the medical periodic table: towards new targets’, Chem. Commun. 2013, 49, 5106–5131.
- 63T. Boulikas, ‘Molecular mechanisms of cisplatin and its liposomally encapsulated form, LipoplatinTM. LipoplatinTM as a chemotherapy and antiangiogenesis drug’, Cancer Ther. 2007, 5, 351–376.
- 64C. S. Allardyce, P. J. Dyson, ‘Metal-based drugs that break the rules’, Dalton Trans. 2016, 45, 3201–3209.
- 65H. Shi, Q. Wang, V. Venkatesh, G. Feng, L. S. Young, Romero-Canelón, I. Zeng, M. P. J. Sadler, ‘Photoactive platinum (IV) complex conjugated to a cancer-cell-targeting cyclic peptide’, Dalton Trans. 2019, 48, 8560–8564.
- 66H. Shi, I. Romero-Canelón, M. Hreusova, O. Novakova, V. Venkatesh, A. Habtemariam, G. J. Clarkson, J. I. Song, V. Brabec, P. J. Sadler, ‘Photoactivatable cell-selective dinuclear trans-diazidoplatinum (IV) anticancer prodrugs’, Inorg. Chem. 2018, 57, 14409–14420.
- 67P. Štarha, B. Drahoš, R. Herchel, ‘An unexpected in-solution instability of diiodido analog of picoplatin complicates its biological characterization’, Dalton Trans. 2021, 50, 6071–6075.
- 68N. Avramović, N. Ignjatović, A. Savić, ‘Kompleksi platine i rutenijuma-obećavajući molekuli u terapiji karcinoma’, Srp. Arh. Celok. Lek. 2019, 147, 107–111.
- 69G. V. Suárez-Moreno, D. Hernández-Romero, Ó. García-Barradas, Ó. Vázquez-Vera, S. Rosete-Luna, C. A. Cruz-Cruz, A. López-Monteon, J. Carrillo-Ahumada, D. Morales-Morales, R. Colorado-Peralta, ‘Second and third-row transition metal compounds containing benzimidazole ligands: An overview of their anticancer and antitumor activity’, Coord. Chem. Rev. 2022, 472, 214790.
- 70C. E. Montañez Giménez, ‘Compuestos antitumorales basados en metales distintos del platino’, 2021.
- 71A. V. Vargiu, A. Robertazzi, A. Magistrato, P. Ruggerone, P. Carloni, ‘The hydrolysis mechanism of the anticancer ruthenium drugs NAMI-A and ICR investigated by DFT-PCM calculations’, J. Phys. Chem. 2008, 112, 4401–4409.
- 72E. Alessio, L. Messori, ‘NAMI-A and KP1019/1339, two iconic ruthenium anticancer drug candidates face-to-face: a case story in medicinal inorganic chemistry’, Molecules 2019, 24, 1995.
- 73R. Trondl, P. Heffeter, C. R. Kowol, M. A. Jakupec, W. Berger, B. K. Keppler, ‘NKP-1339, the first ruthenium-based anticancer drug on the edge to clinical application’, Chem. Sci. 2014, 5, 2925–2932.
- 74K. M. Mahmud, M. S. Niloy, M. S. Shakil, M. A. Islam, ‘Ruthenium Complexes: An Alternative to Platinum Drugs in Colorectal Cancer Treatment’, Pharmaceutica 2021, 13, 1295.
- 75E. Alessio, L. Messori, ‘The deceptively similar ruthenium (III) drug candidates KP1019 and NAMI-A have different actions. what did we learn in the past 30 years’, Metallo-Drugs: Development and Action of Anticancer Agents 2018, 18, 9783110470734-005.
- 76M. Jakubaszek, B. Goud, S. Ferrari, G. Gasser, ‘Mechanisms of action of Ru (II) polypyridyl complexes in living cells upon light irradiation’, Chem. Commun. 2018, 54, 13040–13059.
- 77S. Chatterjee, S. Kundu, A. Bhattacharyya, C. G. Hartinger, P. J. Dyson, ‘The ruthenium (II)-arene compound RAPTA-C induces apoptosis in EAC cells through mitochondrial and p53-JNK pathways’, J. Biol. Inorg. Chem. 2008, 13, 1149–1155.
- 78M. Rausch, P. J. Dyson, P. Nowak-Sliwinska, ‘Recent considerations in the application of RAPTA-C for cancer treatment and perspectives for its combination with immunotherapies’, Adv. Ther. 2019, 2, 1900042.
- 79M. Azmanova, A. Pitto-Barry, ‘Oxidative stress in cancer therapy: Friend or enemy?’, ChemBioChem 2022, 23, e202100641.
- 80M. I. Murillo, C. Gaiddon, R. Le Lagadec, ‘Targeting of the intracellular redox balance by metal complexes towards anticancer therapy’, Front. Chem. 2022, 10, 967337.
- 81A. Kondratskyi, K. Kondratska, F. Vanden Abeele, D. Gordienko, C. Dubois, R. A. Toillon, C. Lemière, S. Slomianny, P. Delcourt, E. Dewailly, ‘Ferroquine, the next generation antimalarial drug, has antitumor activity’, Sci. Rep. 2017, 7, 1–15.
- 82M. Patra, G. Gasser, ‘The medicinal chemistry of ferrocene and its derivatives’, Nat. Chem. Rev. 2017, 1, 1–12.
- 83S. Hoffe, J. Frakes, T. Aguilera, B. Czito, M. Palta, M. Brookes, C. Schweizer, L. Colbert, S. Moningi, M. Bhutani, ‘Randomized, double-blinded, placebo-controlled multicenter adaptive phase 1–2 trial of GC 4419, a dismutase mimetic, in combination with high dose stereotactic body radiation therapy (SBRT) in locally advanced pancreatic cancer (PC)’, Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1399–1400.
- 84S. T. Sonis, ‘Superoxide dismutase as an intervention for radiation therapy-associated toxicities: Review and profile of avasopasem manganese as a treatment option for radiation-induced mucositis’, Drug Des. Dev. Ther. 2021, 15, 1021.
- 85R. W. Orrell, ‘AEOL-10150 (Aeolus)’, Curr. Opin. Invest. Drugs 2006, 7, 70–80.
- 86P. J. Barnes, ‘Oxidative stress-based therapeutics in COPD’, Redox Biol. 2020, 33, 101544.
- 87R. Baskaran, J. Lee, S. G. Yang, ‘Clinical development of photodynamic agents and therapeutic applications’, Biomaterials 2018, 22, 1–8.
10.1186/s40824-017-0112-8 Google Scholar
- 88J. Karges, ‘Klinische Entwicklung von Metallkomplexen als Photosensibilisatoren für die photodynamische Therapie von Krebs’, Angew. Chem. 2022, 134, e202112236.
10.1002/ange.202112236 Google Scholar
- 89M. R. Hamblin, ‘Photodynamic therapy for cancer: what's past is prologue’, Photochem. Photobiol. 2020, 96, 506–516.
- 90D. Cirri, M. G. Fabbrini, A. Pratesi, L. Ciofi, L. Massai, T. Marzo, L. Messori, ‘The leading established metal-based drugs: a revisitation of their relevant physico-chemical data’, BioMetals 2019, 32, 813–817.
- 91P. L. Wood, M. A. Khan, J. R. Moskal, ‘Mechanism of action of the disease-modifying anti-arthritic thiol agents D-penicillamine and sodium aurothiomalate: restoration of cellular free thiols and sequestration of reactive aldehydes’, Eur. J. Pharmacol. 2008, 580, 48–54.
- 92T. Gamberi, G. Chiappetta, T. Fiaschi, A. Modesti, F. Sorbi, F. Magherini, ‘Upgrade of an old drug: Auranofin in innovative cancer therapies to overcome drug resistance and to increase drug effectiveness’, Med. Res. Rev. 2022, 42, 1111–1146.
- 93A. Marín-Hernández, J. C. Gallardo-Pérez, S. Y. López-Ramírez, J. D. García-García, J. S. Rodríguez-Zavala, L. Ruiz-Ramírez, I. Gracia-Mora, A. Zentella-Dehesa, M. Sosa-Garrocho, M. Macías-Silva, ‘Casiopeina II-gly and bromo-pyruvate inhibition of tumor hexokinase, glycolysis, and oxidative phosphorylation’, Arch. Toxicol. 2012, 86, 753–766.
- 94D. X. Robledo-Cadena, J. C. Gallardo-Pérez, V. Dávila-Borja, S. C. Pacheco-Velázquez, J. A. Belmont-Díaz, S. J. Ralph, B. A. Blanco-Carpintero, R. Moreno-Sánchez, S. Rodríguez-Enríquez, ‘Non-steroidal anti-inflammatory drugs increase cisplatin, paclitaxel, and doxorubicin efficacy against human cervix cancer cells’, Pharmaceuticals 2020, 13, 463.
- 95C. Igarashi, H. Matsumoto, M. Takahashi, F. Hihara, T. Tachibana, M. R. Zhang, H. Kurihara, T. Higashi, Y. Yoshii, ‘Identification and quantitative structure-activity relationship assessment of trace chemical impurities contained in the therapeutic formulation of [64Cu] Cu-ATSM’, Nucl. Med. Biol. 2022, 108, 10–15.
- 96D. A. Da Silva, A. De Luca, R. Squitti, M. Rongioletti, L. Rossi, C. M. Machado, G. Cerchiaro, ‘Copper in tumors and the use of copper-based compounds in cancer treatment’, J. Inorg. Biochem. 2022, 226, 111634.
- 97F. Xie, W. Wei, ‘[64Cu] Cu-ATSM: an emerging theranostic agent for cancer and neuroinflammation’, Eur. J. Nucl. Med. Mol. Imaging 2022, 22, 1–9.
- 98R. N. Duffin, V. L. Blair, L. Kedzierski, P. C. Andrews, ‘Alkyl gallium (III) quinolinolates: A new class of highly selective anti-leishmanial agents’, Eur. J. Med. Chem. 2020, 186, 111895.
- 99J. Qi, K. Qian, L. Tian, Z. Cheng, Y. Wang, ‘Gallium (iii)-2-benzoylpyridine-thiosemicarbazone complexes promote apoptosis through Ca 2+ signaling and ROS-mediated mitochondrial pathways’, New J. Chem. 2018, 42, 10226–10233.
- 100A. M. Pizarro, P. J. Sadler, ‘Metal Ion-Nucleic Acid Interactions in Disease and Medicine; In Nucleic Acid-Metal Ion Interactions’, R. Soc. Chem. 2008, 350–416.
- 101I. Bratsos, D. Urankar, E. Zangrando, P. Genova-Kalou, J. Košmrlj, E. Alessio, I. Turel, ‘1-(2-Picolyl)-substituted 1, 2, 3-triazole as novel chelating ligand for the preparation of ruthenium complexes with potential anticancer activity’, Dalton Trans. 2011, 40, 5188–5199.
- 102A. Notaro, A. Frei, R. Rubbiani, M. Jakubaszek, U. Basu, S. Koch, C. Mari, M. Dotou, O. Blacque, J. Gouyon, ‘Ruthenium (II) complex containing a redox-active semiquinonate ligand as a potential chemotherapeutic agent: from synthesis to In Vivo studies’, J. Med. Chem. 2020, 63, 5568–5584.
- 103L. Côrte-Real, F. Mendes, J. Coimbra, T. S. Morais, A. I. Tomaz, A. Valente, M. H. Garcia, I. Santos, M. Bicho, F. Marques, ‘Anticancer activity of structurally related ruthenium (II) cyclopentadienyl complexes’, J. Biol. Inorg. Chem. 2014, 19, 853–867.
- 104S. Ramírez-Rivera, S. Pizarro, M. Gallardo, F. Gajardo, A. Delgadillo, E. De La Fuente-Ortega, F. MacDonnell, G. Bernal, ‘Anticancer activity of two novel ruthenium compounds in gastric cancer cells’, Life Sci. 2018, 213, 57–65.
- 105C. R. Cardoso, M. R. V. Lima, J. Cheleski, E. J. Peterson, T. Venâncio, N. P. Farrell, R. M. Carlos, ‘Luminescent ruthenium complexes for theranostic applications’, J. Med. Chem. 2014, 57, 4906–4915.
- 106J. Yellol, S. A. Perez, A. Buceta, G. Yellol, A. Donaire, P. Szumlas, P. J. Bednarski, G. Makhloufi, C. Janiak, A. Espinosa, C. Novel, ‘N-cyclometalated benzimidazole ruthenium (II) and iridium (III) complexes as antitumor and antiangiogenic agents: a structure-activity relationship study’, J. Med. Chem. 2015, 58, 7310–7327.
- 107T. Moreira, R. Francisco, E. Comsa, S. Duban-Deweer, V. Labas, A. P. Teixeira-Gomes, L. Combes-Soia, F. A. Marques, ‘Matos, Favrelle, A. Polymer ‘ruthenium-cyclopentadienyl’ conjugates-New emerging anticancer drugs’, Eur. J. Med. Chem. 2019, 168, 373–384.
- 108R. Pettinari, Marchetti, F. F. Condello, C. Pettinari, G. Lupidi, R. Scopelliti, S. Mukhopadhyay, T. Riedel, P. J. Dyson, ‘Ruthenium (II)-arene RAPTA type complexes containing curcumin and bisdemethoxycurcumin display potent and selective anticancer activity’, Organometallics 2014, 33, 3709–3715.
- 109A. Kurzwernhart, W. Kandioller, S. Bächler, C. Bächler, S. Martic, M. Buczkowska, G. Mühlgassner, M. A. Jakupec, H. B. Kraatz, P. J. Bednarski, ‘Structure-activity relationships of targeted RuII (η6-p-Cymene) anticancer complexes with flavonol-derived ligands’, J. Med. Chem. 2012, 55, 10512–10522.
- 110Y. Xia, Q. Chen, X. Qin, D. Sun, J. Zhang, J. Liu, ‘Studies of ruthenium(ii)-2,2′-bisimidazole complexes on binding to G-quadruplex DNA and inducing apoptosis in HeLa cells’, New J. Chem. 2013, 37, 3706–3715.
- 111Y. Zhang, L. Lai, P. Cai, G. Z. Cheng, X. M. Xu, Y. Liu, ‘Synthesis, characterization and anticancer activity of dinuclear ruthenium (ii) complexes linked by an alkyl chain’, New J. Chem. 2015, 39, 5805–5812.
- 112B. J. Han, G. B. Jiang, J. Wang, W. Li, H. L. Huang, Y. J. Liu, ‘The studies on bioactivity in vitro of ruthenium (ii) polypyridyl complexes towards human lung carcinoma A549 cells’, RSC Adv. 2014, 4, 40899–40906.
- 113N. Muhammad, N. Sadia, C. Zhu, C. Luo, Z. Guo, X. Wang, ‘Biotin-tagged platinum (iv) complexes as targeted cytostatic agents against breast cancer cells’, Chem. Commun. 2017, 53, 9971–9974.
- 114J. Zhao, W. Hua, G. Xu, S. Gou, ‘Biotinylated platinum (IV) complexes designed to target cancer cells’, J. Inorg. Biochem. 2017, 176, 175–180.
- 115J. J. Zhang, C. M. Che, I. Ott, ‘Caffeine derived platinum (II) N-heterocyclic carbene complexes with multiple anticancer activities’, J. Organomet. Chem. 2015, 782, 37–41.
- 116S. Theiner, H. P. Varbanov, M. Galanski, A. E. Egger, W. Berger, P. Heffeter, B. K. Keppler, ‘Comparative In Vitro and In Vivo pharmacological investigation of platinum (IV) complexes as novel anticancer drug candidates for oral application’, J. Biol. Inorg. Chem. 2015, 20, 89–99.
- 117H. P. Varbanov, S. Göschl, P. Heffeter, S. Theiner, A. Roller, F. Jensen, M. A. Jakupec, W. Berger, Galanski, M. S. B. K. Keppler, ‘A novel class of bis-and tris-chelate diam (m) inebis (dicarboxylato) platinum (IV) complexes as potential anticancer prodrugs’, J. Med. Chem. 2014, 57, 6751–6764.
- 118W. Liu, J. Jiang, Y. Xu, S. Hou, L. Sun, Q. Ye, L. Lou, ‘Design, synthesis and anticancer activity of diam (m) ine platinum (II) complexes bearing a small-molecular cell apoptosis inducer dichloroacetate’, J. Inorg. Biochem. 2015, 146, 14–18.
- 119R. W. Y. Sun, A. L. F. Chow, X. H. Li, J. J. Yan, S. S. Y. Chui, C. M. Che, ‘Luminescent cyclometalated platinum (II) complexes containing N-heterocyclic carbene ligands with potent In Vitro and In Vivo anticancer properties accumulate in cytoplasmic structures of cancer cells’, Chem. Sci. 2011, 2, 728–736.
- 120Y. Y. Scaffidi-Domianello, A. A. Legin, M. A. Jakupec, A. Roller, V. Y. Kukushkin, M. S. Galanski, B. K. Keppler, ‘Novel oximato-bridged platinum (II) Di-and trimer (s): synthetic, structural, and In Vitro anticancer activity studies’, Inorg. Chem. 2012, 51, 7153–7163.
- 121Q. P. Qin, S. L. Wang, M. X. Tan, Z. F. Wang, D. M. Luo, B. Q. Zou, Y. C. Liu, P. F. Yao, H. Liang, ‘Novel tacrine platinum (II) complexes display high anticancer activity via inhibition of telomerase activity, dysfunction of mitochondria, and activation of the p53 signaling pathway’, Eur. J. Med. Chem. 2018, 158, 106–122.
- 122G. Dahm, C. Bailly, L. Karmazin, S. Bellemin-Laponnaz, ‘Synthesis, structural characterization and In Vitro anticancer activity of functionalized N-heterocyclic carbene platinum and palladium complexes’, J. Organomet. Chem. 2015, 794, 115–124.
- 123J. Parker, M. Devocelle, M. Morgan, C. Marmion, ‘Derivatisation of buforin IIb, a cationic henicosapeptide, to afford its complexation to platinum (ii) resulting in a novel platinum (ii)-buforin IIb conjugate with anticancer activity’, Dalton Trans. 2016, 45, 13038–13041.
- 124A. C. Gonçalves, T. S. Morais, M. P. Robalo, F. Marques, F. Avecilla, C. P. Matos, I. Santos, A. I. Tomaz, M. H. Garcia, ‘Important cytotoxicity of novel iron (II) cyclopentadienyl complexes with imidazole based ligands’, J. Inorg. Biochem. 2013, 129, 1–8.
- 125M. Gonzalez-Bartulos, C. Aceves-Luquero, J. Qualai, O. Cusso, M. A. Martínez, S. Fernandez de Mattos, J. A. Menendez, P. Villalonga, M. Costas, X. Ribas, ‘Pro-oxidant activity of amine-pyridine-based iron complexes efficiently kills cancer and cancer stem-like cells’, PLoS One 2015, 10, e0137800.
- 126F. Shabani, L. A. Saghatforoush, S. Ghammamy, ‘Synthesis, characterization and anti-tumor activity of Iron (III) Schiff base complexes with unsymmetric tetradentate ligands’, Bull. Chem. Soc. Ethiop. 2010, 24, 193–199.
- 127T. Sarkar, R. J. Butcher, S. Banerjee, S. Mukherjee, A. Hussain, ‘Visible light-induced cytotoxicity of a dinuclear iron (III) complex of curcumin with low-micromolar IC50 value in cancer cells’, Inorg. Chim. Acta 2016, 439, 8–17.
- 128S. Ramakrishnan, E. Suresh, A. Riyasdeen, M. A. Akbarsha, ‘Palaniandavar, M. DNA binding, prominent DNA cleavage and efficient anticancer activities of tris (diimine) iron (II) complexes’, Dalton Trans. 2011, 40, 3524–3536.
- 129J. Vančo, Z. Šindelář, Z. Dvořák, Z. Trávníček, ‘Iron-salophen complexes involving azole-derived ligands: A new group of compounds with high-level and broad-spectrum in vitro antitumor activity’, J. Inorg. Biochem. 2015, 142, 92–100.
- 130L. Oehninger, L. N. Küster, C. Schmidt, A. Muñoz-Castro, A. Prokop, I. Ott, ‘A Chemical-Biological Evaluation of Rhodium (I) N-Heterocyclic Carbene Complexes as Prospective Anticancer Drugs’, Eur. J. Chem. 2013, 19, 17871–17880.
- 131O. Dömötör, S. Aicher, M. Schmidlehner, M. S. Novak, A. Roller, M. A. Jakupec, W. Kandioller, B. K. Hartinger, C. G. Keppler, É. A. Enyedy, ‘Antitumor pentamethylcyclopentadienyl rhodium complexes of maltol and allomaltol: Synthesis, solution speciation and bioactivity’, J. Inorg. Biochem. 2014, 134, 57–65.
- 132T. M. Khan, N. S. Gul, X. Lu, R. Kumar, M. I. Choudhary, H. Liang, Z. F. Chen, ‘Rhodium (III) complexes with isoquinoline derivatives as potential anticancer agents: In Vitro and In Vivo activity studies’, Dalton Trans. 2019, 48, 11469–11479.
- 133B. P. R. Aradhyula, M. Kalidasan, K. Gangele, D. K. Deb, S. L. Shepherd, R. M. Phillips, K. M. Poluri, M. R. Kollipara, ‘Synthesis, Structural and Biological Studies of Some Half-Sandwich d6-Metal Complexes with Pyrimidine-Based Ligands’, Chem. Select. 2017, 2, 2065–2076.
- 134A. C. Matsheku, M. Y. H. Chen, S. Jordaan, S. Prince, G. S. Smith, B. C. Makhubela, ‘Acridine-containing RuII, OsII, RhIII and IrIII Half-Sandwich Complexes: Synthesis, Structure and Anti-proliferative Activity’, Appl. Organomet. Chem. 2017, 31, e3852.
- 135R. Rubbiani, S. Can, I. Kitanovic, H. Alborzinia, M. Stefanopoulou, M. Kokoschka, S. Mönchgesang, W. S. Sheldrick, S. Wölfl, I. Ott, ‘Comparative in vitro evaluation of N-heterocyclic carbene gold (I) complexes of the benzimidazolylidene type’, J. Med. Chem. 2011, 54, 8646–8657.
- 136W. Liu, K. Bensdorf, M. Proetto, U. Abram, A. Hagenbach, R. Gust, ‘NHC gold halide complexes derived from 4, 5-diarylimidazoles: synthesis, structural analysis, and pharmacological investigations as potential antitumor agents’, J. Med. Chem. 2011, 54, 8605–8615.
- 137C. H. Wang, W. C. Shih, H. C. Chang, Y. Y. Kuo, W. C. Hung, T. G. Ong, W. S. Li, ‘Preparation and characterization of amino-linked heterocyclic carbene palladium, gold, and silver complexes and their use as anticancer agents that act by triggering apoptotic cell death’, J. Med. Chem. 2011, 54, 5245–5249.
- 138A. Casini, M. C. Diawara, R. Scopelliti, S. M. Zakeeruddin, M. Grätzel, P. J. Dyson, ‘Synthesis, characterisation and biological properties of gold (III) compounds with modified bipyridine and bipyridylamine ligands’, Dalton Trans. 2010, 39, 2239–2245.
- 139S. S. Al-Jaroudi, M. Altaf, A. A. Al-Saadi, A. N. Kawde, S. Altuwaijri, S. Ahmad, A. A. Isab, ‘Synthesis, characterization and theoretical calculations of (1, 2-diaminocyclohexane)(1, 3-diaminopropane) gold (III) chloride complexes: in vitro cytotoxic evaluations against human cancer cell lines’, BioMetals 2015, 28, 827–844.
- 140S. T. Chew, K. M. Lo, S. K. Lee, M. P. Heng, W. Y. Teoh, K. S. Sim, K. W. Tan, ‘Copper complexes with phosphonium containing hydrazone ligand: topoisomerase inhibition and cytotoxicity study’, Eur. J. Med. Chem. 2014, 76, 397–407.
- 141J. G. Da Silva, A. A. R. Despaigne, S. R. Louro, C. C. Bandeira, E. M. Souza-Fagundes, H. Beraldo, ‘Cytotoxic activity, albumin and DNA binding of new copper (II) complexes with chalcone-derived thiosemicarbazones’, Eur. J. Med. Chem. 2013, 65, 415–426.
- 142D. Palanimuthu, S. V. Shinde, K. Somasundaram, A. G. Samuelson, ‘In Vitro and In Vivo anticancer activity of copper bis (thiosemicarbazone) complexes’, J. Med. Chem. 2013, 56, 722–734.
- 143Y. Gou, J. Li, B. Fan, B. Xu, M. Zhou, F. Yang, ‘Structure and biological properties of mixed-ligand Cu (II) Schiff base complexes as potential anticancer agents’, Eur. J. Med. Chem. 2017, 134, 207–217.
- 144G. Y. Li, K. J. Du, J. Q. Wang, J. W. Liang, J. F. Kou, X. J. Hou, L. N. Ji, H. Chao, ‘Synthesis, crystal structure, DNA interaction and anticancer activity of tridentate copper (II) complexes’, J. Inorg. Biochem. 2013, 119, 43–53.
- 145R. Govindarajan, R. Nagarajaprakash, V. Veena, N. Sakthivel, B. Manimaran, ‘One-pot reaction of amide functionalized Re (I) based dinuclear metallacycles: Synthesis, characterization and evaluation for anticancer potential’, Polyhedron 2018, 139, 229–236.
- 146S. C. Marker, A. P. King, S. Granja, B. Vaughn, J. J. Woods, E. Boros, J. Wilson, ‘Exploring the In Vivo and In Vitro anticancer activity of rhenium isonitrile complexes’, Inorg. Chem. 2020, 59, 10285–10303.
- 147C. A. Kumar, S. Karthikeyan, B. Varghese, V. Veena, N. Sakthivel, B. Manimaran, ‘Synthesis, characterisation and cytotoxicity evaluation of rhenium (I) based ester functionalised dinuclear metallacyclophanes’, J. Organomet. Chem. 2014, 766, 86–94.
- 148J. Delasoie, A. Pavic, N. Voutier, S. Vojnovic, A. Crochet, J. Nikodinovic-Runic, F. Zobi, ‘Highly Potent rhenium (I) Tricarbonyl Complexes with Dual Anticancer and Anti-Angiogenic Activity Against Colorectal Carcinoma’, 2020.
- 149K. Suntharalingam, S. G. Awuah, P. M. Bruno, T. C. Johnstone, F. Wang, W. Lin, Y. R. Zheng, J. E. Page, M. T. Hemann, S. Lippard, ‘Necroptosis-inducing rhenium (V) oxo complexes’, J. Am. Chem. Soc. 2015, 137, 2967–2974.
- 150L. Reytman, J. Hochman, E. Tshuva, ‘Anticancer diaminotris (phenolato) vanadium (V) complexes: Ligand-metal interplay’, J. Coord. Chem. 2018, 71, 2003–2011.
- 151L. Ni, H. Zhao, L. Tao, X. Li, Z. Zhou, Y. Sun, C. Chen, D. Wei, Y. Liu, G. Diao, ‘Synthesis, In Vitro cytotoxicity, and structure-activity relationships (SAR) of multidentate oxidovanadium (IV) complexes as anticancer agents’, Dalton Trans. 2018, 47, 10035–10045.
- 152I. Correia, S. Roy, C. P. Matos, S. Borovic, N. Butenko, I. Cavaco, F. Marques, J. Lorenzo, A. Rodríguez, V. J. J. O. I. B. Moreno, ‘Vanadium (IV) and copper (II) complexes of salicylaldimines and aromatic heterocycles: Cytotoxicity, DNA binding and DNA cleavage properties’, J. Inorg. Biochem. 2015, 147, 134–146.
- 153Y. L. Bai, Y. W. Zhang, J. Y. Xiao, H. W. Guo, X. W. Liao, W. J. Li, Y. Zhang, ‘Oxovanadium phenanthroimidazole derivatives: synthesis, DNA binding and antitumor activities’, Transition Met. Chem. 2018, 43, 171–183.
- 154P. Ying, P. Zeng, J. Lu, H. Chen, X. Liao, N. Yang, ‘Design, D. New Oxidovanadium Complexes Incorporating Thiosemicarbazones and 1, 10-Phenanthroline Derivatives as DNA Cleavage, Potential Anticancer Agents, and Hydroxyl Radical Scavenger’, Chem. Biol. Drug Des. 2015, 86, 926–937.
- 155J. J. M. Medina, L. G. Naso, A. L. Pérez, A. Rizzi, N. B. Okulik, E. G. Ferrer, P. Williams, P. A. Chemistry, ‘Apigenin oxidovanadium (IV) cation interactions. Synthesis, spectral, bovine serum albumin binding, antioxidant and anticancer studies’, J. Photochem. Photobiol. 2017, 344, 84–100.
- 156J. Li, R. Liu, J. Jiang, X. Liang, L. Huang, G. Huang, H. Chen, L. Pan, Z. Ma, ‘Zinc (II) terpyridine complexes: Substituent effect on photoluminescence, anti-proliferative activity, and DNA interaction’, Mol. 2019, 24, 4519.
- 157C. P. Matos, Y. Addis, P. Nunes, S. Barroso, I. Alho, M. Martins, A. P. Matos, F. Marques, I. Cavaco, J. Pessoa, ‘Exploring the cytotoxic activity of new phenanthroline salicylaldimine Zn (II) complexes’, J. Inorg. Biochem. 2019, 198, 110727.
- 158E. Apohan, U. Yilmaz, O. Yilmaz, A. Serindag, H. Küçükbay, O. Yesilada, Y. Baran, ‘Synthesis, cytotoxic and antimicrobial activities of novel cobalt and zinc complexes of benzimidazole derivatives’, J. Organomet. Chem. 2017, 828, 52–58.
- 159H. Amouri, J. Moussa, A. K. Renfrew, P. J. Dyson, M. N. Rager, L. Chamoreau, ‘Discovery, structure, and anticancer activity of an iridium complex of diselenobenzoquinone’, Angew. Chem. Int. Ed. 2010, 49, 7530–7533.
- 160H. Liu, X. Ge, X. Zhao, Y. Tian, X. Ren, T. Wang, Y. Zhao, Z. Liu, ‘Half-sandwich iridium (III) complexes with α-picolinic acid frameworks and antitumor applications’, J. Inorg. Biochem. 2019, 192, 52–61.
- 161Y. Xie, S. Zhang, X. Ge, W. Ma, X. He, Y. Zhao, J. Ye, H. Zhang, A. Wang, Z. Liu, ‘Lysosomal-targeted anticancer half-sandwich iridium (III) complexes modified with lonidamine amide derivatives’, Appl. Organomet. Chem. 2020, 34, e5589.
- 162Z. D. Mou, N. Deng, F. Zhang, J. Zhang, J. Cen, X. Zhang, ‘Half-sandwich’ Schiff-base Ir (III) complexes as anticancer agents’, Eur. J. Med. Chem. 2017, 138, 72–82.
- 163J. Li, Z. Tian, Z. Xu, S. Zhang, Y. Feng, L. Zhang, Z. Liu, ‘Highly potent half-sandwich iridium and ruthenium complexes as lysosome-targeted imaging and anticancer agents’, Dalton Trans. 2018, 47, 15772–15782.
- 164Q. Du, L. Guo, X. Ge, L. Zhao, Z. Tian, X. Liu, F. Zhang, Z. Liu, ‘Serendipitous synthesis of five-coordinated half-sandwich aminoimine iridium (iii) and ruthenium (ii) complexes and their application as potent anticancer agents’, Inorg. Chem. 2019, 58, 5956–5965.
- 165N. Kumar, R. Kaushal, A. Chaudhary, S. Arora, P. J. I. Awasthi, N. M. Chemistry, ‘Titanium based mixed ligand complexes: Synthesis, spectroscopic and in vitro anti-proliferative studies’, Inorg. Chem. 2018, 48, 467–476.
- 166R. Kaushal, N. Kumar, A. Chaudhary, S. Arora, P. Awasthi, ‘Applications, Synthesis, spectral characterization, and anti-proliferative studies of mixed ligand titanium complexes of adamantylamine’, Bioinorg. Chem. Appl. 2014, 2014, 1–12.
- 167C. E. Saturnino, A. Sirignano, M. S. Botta, A. Sinicropi, A. Caruso, R. Pisano, M. Lappano, P. Maggiolini, M. C. Longo, ‘New titanocene derivatives with high anti-proliferative activity against breast cancer cells’, Bioinorg. Chem. Appl. 2014, 24, 136–140.
- 168R. Serrano, I. Martinez-Argudo, M. Fernandez-Sanchez, P. J. Pacheco-Liñan, I. Bravo, B. Cohen, R. Calero, M. Ruiz, ‘New titanocene derivative with improved stability and binding ability to albumin exhibits high anticancer activity’, J. Inorg. Biochem. 2021, 223, 111562.
- 169M. X. Li, M. Yang, J. Y. Niu, L. Z. Zhang, S. Q. Xie, ‘A nine-coordinated bismuth (III) complex derived from pentadentate 2, 6-diacetylpyridine bis (4 N-methylthiosemicarbazone): crystal structure and both in vitro and in vivo biological evaluation’, Inorg. Chem. 2012, 51, 12521–12526.
- 170R. Ouyang, Y. Yang, X. Tong, K. Feng, Y. Yang, H. Tao, X. Zhang, T. Zong, P. Cao, F. Xiong, ‘Potent anticancer activity of a new bismuth (III) complex against human lung cancer cells’, J. Inorg. Biochem. 2017, 168, 18–26.
- 171Y. Fang, Y. T. Wang, M. Zhao, Y. L. Lu, M. X. Li, Y. H. Zhang, ‘Bismuth (III) and diorganotin (IV) complexes of bis (2-acetylpyridine) thiocarbonohydrazone: synthesis, characterization, and apoptosis mechanism of action In Vitro’, Polyhedron 2018, 155, 254–260.
- 172A. D. M. Mohamad, M. Abualreish, A. M. Abu-Dief, ‘Antimicrobial and anticancer activities of cobalt (III)-hydrazone complexes: Solubilities and chemical potentials of transfer in different organic co-solvent-water mixtures’, J. Mol. Liq. 2019, 290, 111162.
- 173M. R. Kaluđerović, S. Gómez-Ruiz, B. Gallego, E. Hey-Hawkins, R. Paschke, G. N. Kaluđerović, ‘Anticancer activity of dinuclear gallium (III) carboxylate complexes’, Eur. J. Med. Chem. 2010, 45, 519–525.
- 174W. Cao, J. Q. Qian, K. L. Tian, Z. Cheng, Y. Wang, ‘Structure-activity relationships of 2-quinolinecarboxaldehyde thiosemicarbazone gallium (III) complexes with potent and selective anticancer activity’, J. Inorg. Biochem. 2019, 191, 174–182.
- 175M. A. Hussein, T. S. Guan, R. A. Haque, M. B. K. Ahamed, A. M. A. Majid, ‘Synthesis and characterization of thiosemicarbazonato molybdenum (VI) complexes: In vitro DNA binding, cleavage, and antitumor activities’, Polyhedron 2015, 85, 93–103.
- 176I. Berasaluce, K. Cseh, A. Roller, M. Hejl, P. Heffeter, W. Berger, M. A. Jakupec, W. Kandioller, M. S. Malarek, B. K. Keppler, ‘The First Anticancer Tris (pyrazolyl) borate Molybdenum (IV) Complexes: Tested In Vitro and In Vivo – A Comparison of O, O-, S, O-, and N, N-Chelate Effects’, Eur. J. Chem. 2020, 26, 2211–2221.
- 177S. Şahin-Bölükbaşı, N. Şahin, ‘Novel Silver-NHC complexes: Synthesis and anticancer properties’, J. Organomet. Chem. 2019, 891, 78–84.
- 178H. A. Mohamed, B. R. Lake, T. Laing, R. M. Phillips, C. E. Willans, ‘Synthesis and anticancer activity of silver (I)-N-heterocyclic carbene complexes derived from the natural xanthine products caffeine, theophylline and theobromine’, Dalton Trans. 2015, 44, 7563–7569.
- 179N. Şahin, S. Şahin-Bölükbaşı, M. N. Tahir, C. Arıcı, E. Cevik, N. Gürbüz, İ. Özdemir, B. S. Cummings, ‘Synthesis, characterization and anticancer activity of allyl substituted N-Heterocyclic carbene silver (I) complexes’, J. Mol. Struct. 2019, 1179, 92–99.
- 180J. Haribabu, K. Jeyalakshmi, Y. Arun, N. S. Bhuvanesh, P. T. Perumal, R. Karvembu, ‘Synthesis, DNA/protein binding, molecular docking, DNA cleavage and in vitro anticancer activity of nickel (II) bis (thiosemicarbazone) complexes’, RSC Adv. 2015, 5, 46031–46049.