Six New Polyhydroxysteroidal Glycosides, Anthenosides S1 – S6, from the Starfish Anthenea sibogae
Alla A. Kicha
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far East Branch of the Russian Academy of Sciences, Pr. 100-let Vladivostoku 159, 690022 Vladivostok, Russia
Search for more papers by this authorDinh T. Ha
Institute of Natural Products Chemistry, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Nighiado, Cau Giay, Hanoi, Vietnam
Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam
Search for more papers by this authorNatalia V. Ivanchina
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far East Branch of the Russian Academy of Sciences, Pr. 100-let Vladivostoku 159, 690022 Vladivostok, Russia
Search for more papers by this authorTimofey V. Malyarenko
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far East Branch of the Russian Academy of Sciences, Pr. 100-let Vladivostoku 159, 690022 Vladivostok, Russia
Search for more papers by this authorAnatoly I. Kalinovsky
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far East Branch of the Russian Academy of Sciences, Pr. 100-let Vladivostoku 159, 690022 Vladivostok, Russia
Search for more papers by this authorPavel S. Dmitrenok
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far East Branch of the Russian Academy of Sciences, Pr. 100-let Vladivostoku 159, 690022 Vladivostok, Russia
Search for more papers by this authorSvetlana P. Ermakova
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far East Branch of the Russian Academy of Sciences, Pr. 100-let Vladivostoku 159, 690022 Vladivostok, Russia
Search for more papers by this authorOlesya S. Malyarenko
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far East Branch of the Russian Academy of Sciences, Pr. 100-let Vladivostoku 159, 690022 Vladivostok, Russia
Search for more papers by this authorNguyen A. Hung
Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam
Search for more papers by this authorTran T. T. Thuy
Institute of Natural Products Chemistry, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Nighiado, Cau Giay, Hanoi, Vietnam
Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam
Search for more papers by this authorPham Q. Long
Institute of Natural Products Chemistry, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Nighiado, Cau Giay, Hanoi, Vietnam
Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam
Search for more papers by this authorAlla A. Kicha
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far East Branch of the Russian Academy of Sciences, Pr. 100-let Vladivostoku 159, 690022 Vladivostok, Russia
Search for more papers by this authorDinh T. Ha
Institute of Natural Products Chemistry, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Nighiado, Cau Giay, Hanoi, Vietnam
Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam
Search for more papers by this authorNatalia V. Ivanchina
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far East Branch of the Russian Academy of Sciences, Pr. 100-let Vladivostoku 159, 690022 Vladivostok, Russia
Search for more papers by this authorTimofey V. Malyarenko
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far East Branch of the Russian Academy of Sciences, Pr. 100-let Vladivostoku 159, 690022 Vladivostok, Russia
Search for more papers by this authorAnatoly I. Kalinovsky
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far East Branch of the Russian Academy of Sciences, Pr. 100-let Vladivostoku 159, 690022 Vladivostok, Russia
Search for more papers by this authorPavel S. Dmitrenok
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far East Branch of the Russian Academy of Sciences, Pr. 100-let Vladivostoku 159, 690022 Vladivostok, Russia
Search for more papers by this authorSvetlana P. Ermakova
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far East Branch of the Russian Academy of Sciences, Pr. 100-let Vladivostoku 159, 690022 Vladivostok, Russia
Search for more papers by this authorOlesya S. Malyarenko
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far East Branch of the Russian Academy of Sciences, Pr. 100-let Vladivostoku 159, 690022 Vladivostok, Russia
Search for more papers by this authorNguyen A. Hung
Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam
Search for more papers by this authorTran T. T. Thuy
Institute of Natural Products Chemistry, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Nighiado, Cau Giay, Hanoi, Vietnam
Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam
Search for more papers by this authorPham Q. Long
Institute of Natural Products Chemistry, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Nighiado, Cau Giay, Hanoi, Vietnam
Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam
Search for more papers by this authorAbstract
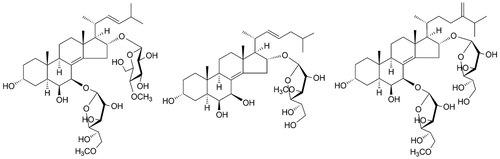
Six new polyhydroxysteroidal glycosides, anthenosides S1 – S6 (1 – 6), along with a mixture of two previously known related glycosides, 7 and 8, were isolated from the methanolic extract of the starfish Anthenea sibogae. The structures of 1 – 6 were established by NMR and HR-ESI-MS techniques as well as by chemical transformations. All new compounds have a 5α-cholest-8(14)-ene-3α,6β,7β,16α-tetrahydroxysteroidal nucleus and differ from majority of starfish glycosides in positions of carbohydrate moieties at C(7) and C(16) (1 – 4, 6) or only at C(16) (5). The 4-O-methyl-β-d-glucopyranose residue (2) and Δ24-cholestane side chain (3) have not been found earlier in the starfish steroidal glycosides. The mixture of 7 and 8 slightly inhibited the proliferation of human breast cancer T-47D cells and decreased the colony size in the colony formation assay.
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under https://doi.org/10.1002/cbdv.201700553.
| Filename | Description |
|---|---|
| cbdv201700553-sup-0001-FigS1-S40.docxWord document, 1.4 MB | |
| cbdv201700553-sup-0002-Supinfo.pdfPDF document, 930.7 KB |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. Minale, R. Riccio, F. Zollo, ‘Steroidal Oligoglycosides and Polyhydroxysteroids from Echinoderms’, Fortschr. Chem. Org. Naturst. 1993, 62, 75 – 308.
- 2V. A. Stonik, ‘Marine Polar Steroids’, Russ. Chem. Rev. 2001, 70, 673 – 715.
- 3M. Iorizzi, S. De Marino, F. Zollo, ‘Steroidal Oligoglycosides from the Asteroidea’, Curr. Org. Chem. 2001, 5, 951 – 973.
- 4V. A. Stonik, N. V. Ivanchina, A. A. Kicha, ‘New Polar Steroids from Starfish’, Nat. Prod. Commun. 2008, 3, 1587 – 1610.
- 5N. V. Ivanchina, A. A. Kicha, V. A. Stonik, ‘Steroid Glycosides from Marine Organisms’, Steroids 2011, 76, 425 – 454.
- 6G. Dong, T. Xu, B. Yang, X. Lin, X. Zhou, X. Yang, Y. Liu, ‘Chemical Constituents and Bioactivities of Starfish’, Chem. Biodiversity 2011, 8, 740 – 791.
- 7N. V. Ivanchina, A. A. Kicha, T. V. Malyarenko, V. A. Stonik, ‘ Recently Studies of Polar Steroids from Starfish: Stuctures, Biological Activities and Biosynthesis’, in ‘ Advances in Natural Products Discovery’, Eds. A. R. Gomes, T. Rocha-Santos and A. Duarte, Nova Science Publishers, Inc., 2017, Chapt. 6, p. 191.
- 8N. Ma, H. F. Tang, F. Qiu, H. W. Lin, X. R. Tian, W. Zhang, ‘A New Polyhydroxysteroidal Glycoside from the Starfish Anthenea chinensis’, Chin. Chem. Lett. 2009, 20, 1231 – 1234.
- 9N. Ma, H.-F. Tang, F. Qiu, H.-W. Lin, X.-R. Tian, M.-N. Yao, ‘Polyhydroxysteroidal Glycosides from the Starfish Anthenea chinensis’, J. Nat. Prod. 2010, 73, 590 – 597.
- 10T. V. Malyarenko, S. D. Kharchenko, A. A. Kicha, N. V. Ivanchina, P. S. Dmitrenok, E. A. Chingizova, E. A. Pislyagin, E. V. Evtushenko, T. I. Antokhina, Ch. V. Minh, V. A. Stonik, ‘Anthenosides L–U, Steroidal Glycosides with Unusual Structural Features from the Starfish Anthenea aspera’, J. Nat. Prod. 2016, 79, 3047 – 3056.
- 11J.-X. Kang, Y.-F. Kang, H. Han, ‘Three New Cytotoxic Polyhydroxysteroidal Glycosides from the Starfish Craspidaster hesperus’, Mar. Drugs 2016, 14, 189 – 200.
- 12D. J. Vanderah, C. Djerassi, ‘Marine Natural Products. Synthesis of Four Naturally Occurring 20β-H Cholanic Acid Derivatives’, J. Org. Chem. 1978, 43, 1442 – 1448.
- 13K. Bock, H. Thøgersen, ‘Nuclear Magnetic Resonance Spectroscopy in the Study of Mono- and Oligosaccharides’, Ann. Rep. NMR Spectrosc. 1983, 13, 1 – 57.
10.1016/S0066-4103(08)60307-5 Google Scholar
- 14S. Dasari, P. B. Tchounwou, ‘Cisplatin in cancer therapy: Molecular mechanisms of action’, Eur. J. Pharmacol. 2014, 740, 364 – 378.
- 15K. Leontein, B. Lindberg, J. Lönngren, ‘Assignment of Absolute Configuration of Sugars by g.l.c. of Their Acetylated Glycosides Formed from Chiral Alcohols’, Carbohydr. Res. 1978, 62, 359 – 362.
- 16E. V. Evtushenko, Y. S. Ovodov, ‘Partial Methylation of methyl α-D-glucopyranoside. 2. Preparative Liquid and Gas-liquid-Chromatography of mono-O-methyl and di-O-methyl Ether Acetates of methyl α-D-glucopyranoside’, Khim. Prir. Soedin. 1982, 1, 21 – 23.
- 17E. V. Evtushenko, E. Y. Plisova, Y. S. Ovodov, ‘Preparative synthesis of methyl-β-D-galactopyranoside methyl ethers’, Bioorg. Khim. 1986, 12, 1366 – 1371.
- 18O. S. Malyarenko, R. V. Usoltseva, N. M. Shevchenko, V. V. Isakov, T. N. Zvyagintseva, S. P. Ermakova, ‘In vitro Anticancer Activity of the Laminarans from Far Eastern Brown Seaweeds and Their Sulfated Derivatives’, J. Appl. Phycol. 2017, 29, 543 – 553.
- 19O. S. Malyarenko, S. A. Dyshlovoy, A. A. Kicha, N. V. Ivanchina, T. V. Malyarenko, C. Bokemeyer, vonAmsberg G., V. A. Stonik, S. P. Ermakova, ‘The Inhibitory Activity of Luzonicosides from the Starfish Echinaster luzonicus against Human Melanoma Cells’, Mar. Drugs 2017, 15, 227 – 238.