Physicochemical studies of globular proteins—Bovine serum albumin, egg albumin, and lysozyme—In some aqueous iodide salts solutions of IA group and cetyltrimethyl ammonium bromide systems
Abstract
Densities (ρ, kg m−3), and viscosities (η, 0.1 kg m−1 s−1) of Bovine Serum Albumin (BSA), Egg Albumin, and Lysozyme in aqueous iodide salts of lithium, sodium, and potassium, along with cationic surfactant-cetyltrimethyl ammonium bromide (CTAB) were measured at a temperature of 303.15 K. The 0.0010–0.0018 g %, w/v of each protein at an interval of 0.0002 mol L−1 in 0.2, 0.4, and 0.8 millimol L−1 of salt and CTAB are studied. Data are used for apparent molar volumes (Vϕ, 10−6 m3 mol−1) and intrinsic viscosities ([η], dL kg−1), respectively. Data are regressed and extrapolated to zero concentrations for ρ0, η0, and 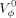 limiting values and Sd, Sη and SV corresponding slopes for protein–salt structural interactions. With size of cations, the densities decrease as CTAB > LiI > NaI > KI and increase with salts concentrations, with salts the densities are as Lysozyme > BSA > Egg Albumin, viscosities and Vϕ as BSA > Egg–Albumin > Lysozyme. The ρ and η values with CTAB higher and [η] are lower and converse at around 0.4 mmol L−1 salt and is effective for greater stability of proteins. The [η] in CTAB are higher than other salts and decreases with size of cations with stronger intermolecular forces. © 2008 Wiley Periodicals, Inc. J Appl Polym Sci, 2008
limiting values and Sd, Sη and SV corresponding slopes for protein–salt structural interactions. With size of cations, the densities decrease as CTAB > LiI > NaI > KI and increase with salts concentrations, with salts the densities are as Lysozyme > BSA > Egg Albumin, viscosities and Vϕ as BSA > Egg–Albumin > Lysozyme. The ρ and η values with CTAB higher and [η] are lower and converse at around 0.4 mmol L−1 salt and is effective for greater stability of proteins. The [η] in CTAB are higher than other salts and decreases with size of cations with stronger intermolecular forces. © 2008 Wiley Periodicals, Inc. J Appl Polym Sci, 2008




