Effect of Ambient Organic Acids on the Water Structure at Interfaces
Graphical Abstract
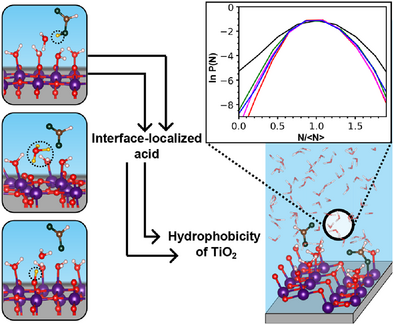
Ambient organic acids such as formic acid tend to localize in the interfacial water layers close to surfaces, facilitated by the interaction and exchange of the acid proton with a surface oxygen. These localized acids make the anatase surface hydrophobic, which is offset by the larger fraction of dissociated water on rutile. Thus, the wettability of is largely controlled by acid-base chemistry rather than chemisorption.
Abstract
A molecular-level understanding of the effects of ambient organic compounds on the wettability of titanium dioxide () surfaces is relevant to many of its energy-related and environmental applications. Herein, we focus on two common atmospheric carboxylic acids, formic and acetic acid, and characterize their adsorption/ desorption at the aqueous interfaces of anatase and rutile using molecular dynamics with an ab initio deep neural network potential. Our simulations show that these acids prefer to be localized in the interfacial water layers close to the surface where they are stabilized by the interaction/exchange of their acid proton with a surface oxygen, rather than chemisorb at the surface Ti sites by displacing the adsorbed water. Notably, these acids make the surface of anatase hydrophobic, whereas the larger fraction of adsorbed water dissociation can offset their effect on rutile. These results provide a picture where carboxylic acids control the wettability of largely through acid-base chemistry at the interface rather than chemisorption on the oxide surface, a finding that can help improve the design of self-cleaning surfaces and photocatalytic devices.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The training dataset, input files used for training, and deep potential models generated in this study are available in the Figshare database and can be accessed using the following link: https://figshare.com/s/308898427f80dc098f7d.