Mechanism Switch Between Radical-Polar Crossover and Radical Buffering
Minghao Xu
College of Chemistry and Molecular Sciences, State Key Laboratory of Power Grid Environmental Protection, Wuhan University, Wuhan, 430072 P.R. China
These authors contributed equally to this work.
Search for more papers by this authorYan-Bo Li
Organisch-Chemisches Institut, Universität Münster, Corrensstraße 40, 48149 Münster, Germany
These authors contributed equally to this work.
Search for more papers by this authorHuamin Wang
Organisch-Chemisches Institut, Universität Münster, Corrensstraße 40, 48149 Münster, Germany
Search for more papers by this authorCorresponding Author
Frank Glorius
Organisch-Chemisches Institut, Universität Münster, Corrensstraße 40, 48149 Münster, Germany
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Xiaotian Qi
College of Chemistry and Molecular Sciences, State Key Laboratory of Power Grid Environmental Protection, Wuhan University, Wuhan, 430072 P.R. China
E-mail: [email protected]; [email protected]
Search for more papers by this authorMinghao Xu
College of Chemistry and Molecular Sciences, State Key Laboratory of Power Grid Environmental Protection, Wuhan University, Wuhan, 430072 P.R. China
These authors contributed equally to this work.
Search for more papers by this authorYan-Bo Li
Organisch-Chemisches Institut, Universität Münster, Corrensstraße 40, 48149 Münster, Germany
These authors contributed equally to this work.
Search for more papers by this authorHuamin Wang
Organisch-Chemisches Institut, Universität Münster, Corrensstraße 40, 48149 Münster, Germany
Search for more papers by this authorCorresponding Author
Frank Glorius
Organisch-Chemisches Institut, Universität Münster, Corrensstraße 40, 48149 Münster, Germany
E-mail: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Xiaotian Qi
College of Chemistry and Molecular Sciences, State Key Laboratory of Power Grid Environmental Protection, Wuhan University, Wuhan, 430072 P.R. China
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
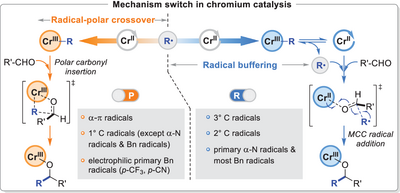
The radical buffering scenario, which features the reversible radical dissociation from alkyl chromium(III) followed by the metal-carbonyl-coupled (MCC) radical addition to form the C─C bond, is established for Cr-catalyzed carbonyl alkylation. This new radical bonding model provides an orthogonal avenue to radical-polar crossover for the design of radical coupling reactions.
Abstract
Radical-polar crossover (RPC) is a classic concept that bridges one- and two-electron chemistry. It has been widely used in Cr-catalyzed carbonyl addition reactions to clarify the formation of alkyl chromium(III) intermediate and subsequent carbonyl insertion. Herein, we proposed an orthogonal bonding model, the radical buffering scenario, for Cr-catalyzed carbonyl alkylation. This radical bonding model features the radical dissociation from the alkyl chromium(III) complex followed by the Cr(II)-carbonyl-coupled radical addition to form the C─C bond. The mechanism switch between the radical and polar bonding models is affected by the radical stability, radical nucleophilicity, radical size, and the presence of an α-heteroatom or α–π bond. The collaborative computational and experimental studies have verified the reliability of the radical mechanism. More importantly, we demonstrated that this radical buffering scenario possesses a different stereoselectivity control model from that in the RPC scenario. A general enantioselectivity and diastereoselectivity control model derived from the multiple ligand-radical interactions is thus established for CrCl2/bisoxazoline-catalyzed asymmetric radical addition.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202500522-sup-0001-SuppMat.docx14.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. Pitzer, J. L. Schwarz, F. Glorius, Chem. Sci. 2019, 10, 8285–8291.
- 2M. Kischkewitz, F. W. Friese, A. Studer, Adv. Synth. Catal. 2020, 362, 2077–2087.
- 3S. Sharma, J. Singh, A. Sharma, Adv. Synth. Catal. 2021, 363, 3146–3169.
- 4Z. Tan, H. Zhang, K. Xu, C. Zeng, Sci. China Chem. 2024, 67, 450–470.
- 5M. Liu, X. Ouyang, C. Xuan, C. Shu, Org. Chem. Front. 2024, 11, 895–915.
- 6J. L. M. Matos, S. Vásquez-Céspedes, J. Gu, T. Oguma, R. A. Shenvi, J. Am. Chem. Soc. 2018, 140, 16976–16981.
- 7K. P. S. Cheung, D. Kurandina, T. Yata, V. Gevorgyan, J. Am. Chem. Soc. 2020, 142, 9932–9937.
- 8W. Zhang, S. Lin, J. Am. Chem. Soc. 2020, 142, 20661–20670.
- 9N. L. Reed, G. A. Lutovsky, T. P. Yoon, J. Am. Chem. Soc. 2021, 143, 6065–6070.
- 10T. Wan, L. Capaldo, G. Laudadio, A. V. Nyuchev, J. A. Rincón, P. García-Losada, C. Mateos, M. O. Frederick, M. Nuño, T. Noël, Angew. Chem. Int. Ed. 2021, 60, 17893–17897.
- 11J. P. Phelan, S. B. Lang, J. S. Compton, C. B. Kelly, R. Dykstra, O. Gutierrez, G. A. Molander, J. Am. Chem. Soc. 2018, 140, 8037–8047.
- 12X. Zhang, W. Wu, W. Cao, H. Yu, X. Xu, X. Liu, X. Feng, Angew. Chem. Int. Ed. 2020, 59, 4846–4850.
- 13S. H. Park, J. Jang, K. Shin, H. Kim, ACS Catal. 2022, 12, 10572–10580.
- 14K. Ota, K. Nagao, D. Hata, H. Sugiyama, Y. Segawa, R. Tokunoh, T. Seki, N. Miyamoto, Y. Sasaki, H. Ohmiya, Nat. Commun. 2023, 14, 6856.
- 15Y. Zhang, S.-S. Chen, K.-D. Li, H.-M. Huang, Angew. Chem. Int. Ed. 2024, 63, e202401671.
- 16H. Zhang, B. Chen, G. Zhang, Org. Lett. 2020, 22, 656–660.
- 17H.-M. Huang, P. Bellotti, F. Glorius, Acc. Chem. Res. 2022, 55, 1135–1147.
- 18Y. Katayama, H. Mitsunuma, M. Kanai, Synthesis 2022, 54, 1684–1694.
- 19Y. Okude, S. Hirano, T. Hiyama, H. Nozaki, J. Am. Chem. Soc. 1977, 99, 3179–3181.
- 20T. Hiyama, K. Kimura, H. Nozaki, Tetrahedron Lett. 1981, 22, 1037–1040.
- 21K. Takai, K. Kimura, T. Kuroda, T. Hiyama, H. Nozaki, Tetrahedron Lett. 1983, 24, 5281–5284.
- 22K. Takai, T. Kuroda, S. Nakatsukasa, K. Oshima, H. Nozakil, Tetrahedron Lett. 1985, 26, 5585–5588.
- 23H. Jin, J. Uenishi, W. J. Christ, Y. Kishi, J. Am. Chem. Soc. 1986, 108, 5644–5646.
- 24K. Takai, K. Nitta, O. Fujimura, K. Utimoto, J. Org. Chem. 1989, 54, 4732–4734.
- 25J. L. Schwarz, H.-M. Huang, T. O. Paulisch, F. Glorius, ACS Catal. 2020, 10, 1621–1627.
- 26H.-M. Huang, P. Bellotti, C. G. Daniliuc, F. Glorius, Angew. Chem. Int. Ed. 2021, 60, 2464–2471.
- 27X. Zeng, F.-H. Zhang, Z. Wang, Org. Chem. Front. 2023, 10, 310–316.
- 28X. Zeng, F.-H. Zhang, R. Lai, X. Lin, Z. Wang, Sci. China Chem. 2024, 67, 1589–1595.
- 29A. Fürstner, Chem. Rev. 1999, 99, 991–1046.
- 30Y. Hirao, Y. Katayama, H. Mitsunuma, M. Kanai, Org. Lett. 2020, 22, 8584–8588.
- 31F. Schäfers, S. Dutta, R. Kleinmans, C. Mück-Lichtenfeld, F. Glorius, ACS Catal. 2022, 12, 12281–12290.
- 32T. Hiyama, Y. Okude, K. Kimura, H. Nozaki, Bull. Chem. Soc. Jpn. 1982, 55, 561–568.
- 33A. Fürstner, N. Shi, J. Am. Chem. Soc. 1996, 118, 12349–12357.
- 34K. Takai, N. Matsukawa, A. Takahashi, T. Fujii, Angew. Chem. Int. Ed. 1998, 37, 152–155.
10.1002/(SICI)1521-3773(19980202)37:1/2<152::AID-ANIE152>3.0.CO;2-8 CAS Web of Science® Google Scholar
- 35D. L. Usanov, H. Yamamoto, Angew. Chem. Int. Ed. 2010, 49, 8169–8172.
- 36H. Mitsunuma, S. Tanabe, H. Fuse, K. Ohkubo, M. Kanai, Chem. Sci. 2019, 10, 3459–3465.
- 37F. Schäfers, L. Quach, J. L. Schwarz, M. Saladrigas, C. G. Daniliuc, F. Glorius, ACS Catal. 2020, 10, 11841–11847.
- 38S. Tanabe, H. Mitsunuma, M. Kanai, J. Am. Chem. Soc. 2020, 142, 12374–12381.
- 39Y. Gao, D. E. Hill, W. Hao, B. J. McNicholas, J. C. Vantourout, R. G. Hadt, S. E. Reisman, D. G. Blackmond, P. S. Baran, J. Am. Chem. Soc. 2021, 143, 9478–9488.
- 40Y. Gao, B. Jiang, N. C. Friede, A. C. Hunter, D. G. Boucher, S. D. Minteer, M. S. Sigman, S. E. Reisman, P. S. Baran, J. Am. Chem. Soc. 2024, 146, 4872–4882.
- 41F.-D. Lu, J. Chen, X. Jiang, J.-R. Chen, L.-Q. Lu, W.-J. Xiao, Chem. Soc. Rev. 2021, 50, 12808–12827.
- 42H. Hu, Z. Shi, X. Guo, F.-H. Zhang, Z. Wang, J. Am. Chem. Soc. 2024, 146, 5316–5323.
- 43J. L. Schwarz, R. Kleinmans, T. O. Paulisch, F. Glorius, J. Am. Chem. Soc. 2020, 142, 2168–2174.
- 44K. Yahata, S. Sakurai, S. Hori, S. Yoshioka, Y. Kaneko, K. Hasegawa, S. Akai, Org. Lett. 2020, 22, 1199–1203.
- 45F.-H. Zhang, X. Guo, X. Zeng, Z. Wang, Nat. Commun. 2022, 13, 5036.
- 46Y. Liu, S. Lin, D. Zhang, B. Song, Y. Jin, E. Hao, L. Shi, Org. Lett. 2022, 24, 3331–3336.
- 47H. Hu, Z. Wang, J. Am. Chem. Soc. 2023, 145, 20775–20781.
- 48H. E. Zimmerman, M. D. Traxler, J. Am. Chem. Soc. 1957, 79, 1920–1923.
- 49J. L. Schwarz, F. Schäfers, A. Tlahuext-Aca, L. Lückemeier, F. Glorius, J. Am. Chem. Soc. 2018, 140, 12705–12709.
- 50Y. Xiong, G. Zhang, J. Am. Chem. Soc. 2018, 140, 2735–2738.
- 51H. Shen, Z. Zhang, Z. Shi, K. Gao, Z. Wang, Chem 2024, 10, 998–1014.
- 52P. Peng, Y. Zhong, C. Zhou, Y. Tao, D. Li, Q. Lu, ACS Cent. Sci. 2023, 9, 756–762.
- 53F.-H. Zhang, X. Guo, X. Zeng, Z. Wang, Angew. Chem. Int. Ed. 2022, 61, e202117114.
- 54X. Guo, Z. Shi, F.-H. Zhang, Z. Wang, ACS Catal. 2023, 13, 3170–3178.
- 55Y.-F. Lv, G. Liu, Z. Shi, Z. Wang, Angew. Chem. Int. Ed. 2024, 63, e202406109.
- 56L. Wessjohann, G. Schmidt, H. Schrekker, Synlett 2007, 2139–2141.
- 57J. K. Kochi, J. W. Powers, J. Am. Chem. Soc. 1970, 92, 137–146.
- 58K. C. MacLeod, J. L. Conway, B. O. Patrick, K. M. Smith, J. Am. Chem. Soc. 2010, 132, 17325–17334.
- 59S. Dutta, J. E. Erchinger, F. Schäfers, A. Das, C. G. Daniliuc, F. Glorius, Angew. Chem. Int. Ed. 2022, 61, e202212136.
- 60Y.-B. Li, M. Xu, L. A. Kellermann, J. E. Erchinger, S. Dutta, C. G. Daniliuc, X. Qi, F. Glorius, J. Am. Chem. Soc. 2025, 147, 2642–2652.
- 61X. Xi, Y. Luo, W. Li, M. Xu, H. Zhao, Y. Chen, S. Zheng, X. Qi, W. Yuan, Angew. Chem. Int. Ed. 2022, 61, e202114731.
- 62F. Ye, S. Zheng, Y. Luo, X. Qi, W. Yuan, ACS Catal. 2024, 14, 8505–8517.
- 63J. Hioe, H. Zipse, Org. Biomol. Chem. 2010, 8, 3609–3617.
- 64L. R. Domingo, P. Pérez, Org. Biomol. Chem. 2013, 11, 4350–4358.
- 65F. Parsaee, M. C. Senarathna, P. B. Kannangara, S. N. Alexander, P. D. E. Arche, E. R. Welin, Nat. Rev. Chem. 2021, 5, 486–499.
- 66S. V. S. Sowndarya, P. C. St John, R. S. Paton, Chem. Sci. 2021, 12, 13158–13166.
- 67J. J. A. Garwood, A. D. Chen, D. A. Nagib, J. Am. Chem. Soc. 2024, 146, 28034–28059.
- 68M. Yuan, Z. Song, S. O. Badir, G. A. Molander, O. Gutierrez, J. Am. Chem. Soc. 2020, 142, 7225–7234.
- 69A. Pulcinella, P. Chandra Tiwari, A. Luridiana, K. Yamazaki, D. Mazzarella, A. K. Sadhoe, A. I. Alfano, E. H. Tiekink, T. A. Hamlin, T. Noël, Angew. Chem. Int. Ed. 2025, 64, e202413846.
- 70D. J. Henry, C. J. Parkinson, P. M. Mayer, L. Radom, J. Phys. Chem. A 2001, 105, 6750–6756.
- 71B. Wang, X. Zhang, Y. Cao, L. Zou, X. Qi, Q. Lu, Angew. Chem. Int. Ed. 2023, 62, e202218179.
- 72X. Xia, Z. Wang, ACS Catal. 2022, 12, 11152–11158.
- 73W.-F. Zheng, J. Chen, X. Qi, Z. Huang, Nat. Chem. 2023, 15, 1672–1682.