Solvent-Controlled Enantiodivergent Construction of P(V)-Stereogenic Molecules via Palladium-Catalyzed Annulation of Prochiral N-Aryl Phosphonamides with Aromatic Iodides
Qingyu Tian
Xiamen Key Laboratory of Optoelectronic Materials and Advanced Manufacturing, College of Materials Science and Engineering, Huaqiao University, Xiamen, 361021 China
Search for more papers by this authorJin Ge
Xiamen Key Laboratory of Optoelectronic Materials and Advanced Manufacturing, College of Materials Science and Engineering, Huaqiao University, Xiamen, 361021 China
Search for more papers by this authorYaopeng Liu
Xiamen Key Laboratory of Optoelectronic Materials and Advanced Manufacturing, College of Materials Science and Engineering, Huaqiao University, Xiamen, 361021 China
Search for more papers by this authorXi Wu
Xiamen Key Laboratory of Optoelectronic Materials and Advanced Manufacturing, College of Materials Science and Engineering, Huaqiao University, Xiamen, 361021 China
Search for more papers by this authorZhenghao Li
Xiamen Key Laboratory of Optoelectronic Materials and Advanced Manufacturing, College of Materials Science and Engineering, Huaqiao University, Xiamen, 361021 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Guolin Cheng
Xiamen Key Laboratory of Optoelectronic Materials and Advanced Manufacturing, College of Materials Science and Engineering, Huaqiao University, Xiamen, 361021 China
Key Lab of Fluorine and Silicon for Energy Materials and Chemistry of Ministry of Education, Jiangxi Normal University, Nanchang, 330022 China
Search for more papers by this authorQingyu Tian
Xiamen Key Laboratory of Optoelectronic Materials and Advanced Manufacturing, College of Materials Science and Engineering, Huaqiao University, Xiamen, 361021 China
Search for more papers by this authorJin Ge
Xiamen Key Laboratory of Optoelectronic Materials and Advanced Manufacturing, College of Materials Science and Engineering, Huaqiao University, Xiamen, 361021 China
Search for more papers by this authorYaopeng Liu
Xiamen Key Laboratory of Optoelectronic Materials and Advanced Manufacturing, College of Materials Science and Engineering, Huaqiao University, Xiamen, 361021 China
Search for more papers by this authorXi Wu
Xiamen Key Laboratory of Optoelectronic Materials and Advanced Manufacturing, College of Materials Science and Engineering, Huaqiao University, Xiamen, 361021 China
Search for more papers by this authorZhenghao Li
Xiamen Key Laboratory of Optoelectronic Materials and Advanced Manufacturing, College of Materials Science and Engineering, Huaqiao University, Xiamen, 361021 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Guolin Cheng
Xiamen Key Laboratory of Optoelectronic Materials and Advanced Manufacturing, College of Materials Science and Engineering, Huaqiao University, Xiamen, 361021 China
Key Lab of Fluorine and Silicon for Energy Materials and Chemistry of Ministry of Education, Jiangxi Normal University, Nanchang, 330022 China
Search for more papers by this authorGraphical Abstract
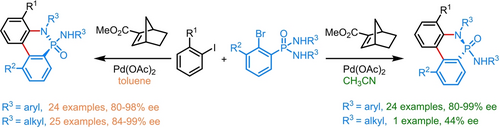
A new strategy for enantiodivergent accessing P(V)-stereogenic molecules via palladium/norbornene cooperative catalysis was established. The enantioselectivity of this protocol was tuned by the polarity of the solvent, thus providing both enantiomers of the P(V)-stereogenic molecules using a single chiral norbornene catalyst.
Abstract
In this work, we describe an efficient and modular method for enantiodivergent accessing P(V)-stereogenic molecules by utilizing the catalytic atroposelective Catellani-type C−H arylation/desymmetric intramolecular N-arylation cascade reaction. The enantioselectivity of this protocol was proved to be tuned by the polarity of the solvent, thus providing a wide range of both chiral P(V)-stereogenic enantiomers in moderate to good yields with good to excellent enantiomeric excesses. Noteworthy is that the strategy developed herein represents an unprecedented example of solvent-dictated inversion of the enantioselectivity of P(V)-stereogenic compounds.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Deposition numbers 2335991 (4 x), contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202409366-sup-0001-misc_information.pdf28.6 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. Kuwabara, Y. Maekawa, T. Murai, Tetrahedron 2020, 76, 131152;
- 1bT. Focken, S. Hanessian, Beilstein J. Org. Chem. 2014, 10, 1848–1877.
- 2J. J. Petkowski, W. Bains, S. Seager, Molecules 2019, 24, 886.
- 3G. P. Horsman, D. L. Zechel, Chem. Rev. 2017, 117, 5704–5783.
- 4
- 4aU. Pradere, E. C. Garnier-Amblard, S. J. Coats, F. Amblard, R. F. Schinazi, Chem. Rev. 2014, 114, 9154–9218;
- 4bY. Mehellou, H. S. Rattan, J. Balzarini, J. Med. Chem. 2018, 61, 2211–2226.
- 5
- 5aR.-Y. Zhu, K. Liao, J.-S. Yu, J. Zhou, Acta Chim. Sin. 2020, 78, 193–216;
- 5bH. Li, L. Yin, Chin. J. Org. Chem. 2022, 42, 3183–3200;
- 5cM. Dutartre, J. Bayardon, S. Juge, Chem. Soc. Rev. 2016, 45, 5771–5794.
- 6
- 6aO. Korpiun, R. A. Lewis, J. Chickos, K. Mislow, J. Am. Chem. Soc. 1968, 90, 4842–4846;
- 6bT. León, A. Riera, X. Verdaguer, J. Am. Chem. Soc. 2011, 133, 5740–5743;
- 6cK. W. Knouse, J. N. deGruyter, M. A. Schmidt, B. Zheng, J. C. Vantourout, C. Kingston, S. E. Mercer, I. M. McDonald, R. E. Olson, Y. Zhu, C. Hang, J. Zhu, C. Yuan, Q. Wang, P. Park, M. D. Eastgate, P. S. Baran, Science 2018, 361, 1234–1238;
- 6dZ. S. Han, N. Goyal, M. A. Herbage, J. D. Sieber, B. Qu, Y. Xu, Z. Li, J. T. Reeves, J.-N. Desrosiers, S. Ma, N. Grinberg, H. Lee, H. P. R. Mangunuru, Y. Zhang, D. Krishnamurthy, B. Z. Lu, J. J. Song, G. Wang, C. H. Senanayake, J. Am. Chem. Soc. 2013, 135, 2474–2477;
- 6eK. Kuwabara, Y. Maekawa, M. Minoura, T. Maruyama, T. Murai, J. Org. Chem. 2020, 85, 14446–14455;
- 6fA. Mondal, N. O. Thiel, R. Dorel, B. L. Feringa, Nat. Catal. 2022, 5, 10–19.
- 7
- 7aQ. Dai, L. Liu, J. Zhang, Angew. Chem. Int. Ed. 2021, 60, 27247–27252; Angew. Chem. 2021, 133, 27453–27458;
- 7bZ.-H. Wu, A.-Q. Cheng, M. Yuan, Y.-X. Zhao, H.-L. Yang, L.-H. Wei, H.-Y. Wang, T. Wang, Z. Zhang, W.-L. Duan, Angew. Chem. Int. Ed. 2021, 60, 27241–27246; Angew. Chem. 2021, 133, 27447–27452;
- 7cC. Wang, X. Hu, C. Xu, Q. Ge, Q. Yang, J. Xiong, W.-L. Duan, Angew. Chem. Int. Ed. 2023, 62, e202300011; Angew. Chem. 2023, 135, e202300011;
- 7dR. Beaud, R. J. Phipps, M. J. Gaunt, J. Am. Chem. Soc. 2016, 138, 13183–13186;
- 7eW.-Q. Cai, Q. Wei, Q.-W. Zhang, Org. Lett. 2022, 24, 1258–1262;
- 7fQ. Dai, W. Li, Z. Lo, J. Zhang, J. Am. Chem. Soc. 2019, 141, 20556–20564;
- 7gX.-T. Liu, Y.-Q. Zhang, X.-Y. Han, S.-P. Sun, Q.-W. Zhang, J. Am. Chem. Soc. 2019, 141, 16584–16589;
- 7hQ. Zhang, X.-T. Liu, Y. Wu, Q.-W. Zhang, Org. Lett. 2021, 23, 8683–8687;
- 7iJ. Kang, K. Ding, S.-M. Ren, B. Su, Angew. Chem. Int. Ed. 2023, 62, e202301628; Angew. Chem. 2023, 135, e202301628.
- 8
- 8aJ.-H. Chen, M.-Y. Teng, F.-R. Huang, H. Song, Z.-K. Wang, H.-L. Zhuang, Y.-J. Wu, X. Wu, Q.-J. Yao, B.-F. Shi, Angew. Chem. Int. Ed. 2022, 61, e202210106; Angew. Chem. 2022, 134, e202301628;
- 8bQ.-J. Yao, J.-H. Chen, H. Song, F.-R. Huang, B.-F. Shi, Angew. Chem. Int. Ed. 2022, 62, e202218533; Angew. Chem. 2022, 135, e202218533;
- 8cT. von Muenchow, S. Dana, Y. Xu, B. Yuan, L. Ackermann, Science 2023, 379, 1036–1042;
- 8dA. Das, R. Mandal, H. S. Ravi Sankar, S. Kumaran, J. R. Premkumar, D. Borah, B. Sundararaju, Angew. Chem. Int. Ed. 2024, 63, e202315005; Angew. Chem. 2024, 136, e202315005;
- 8eZ.-J. Du, J. Guan, G.-J. Wu, P. Xu, L.-X. Gao, F.-S. Han, J. Am. Chem. Soc. 2015, 137, 632–635;
- 8fZ.-Q. Lin, W.-Z. Wang, S.-B. Yan, W.-L. Duan, Angew. Chem. Int. Ed. 2015, 54, 6265–6269; Angew. Chem. 2015, 127, 6363–6367;
- 8gS.-B. Yan, R. Wang, Z.-G. Li, A.-N. Li, C. Wang, W.-L. Duan, Nat. Commun. 2023, 14, 2264;
- 8hL. Liu, A.-A. Zhang, Y. Wang, F. Zhang, Z. Zuo, W.-X. Zhao, C.-L. Feng, W. Ma, Org. Lett. 2015, 17, 2046–2049;
- 8iY.-S. Jang, L. Wozniak, J. Pedroni, N. Cramer, Angew. Chem. Int. Ed. 2018, 57, 12901–12905; Angew. Chem. 2018, 130, 13083–13087;
- 8jC.-Y. Wang, T. Zhou, B.-F. Shi, ACS Catal. 2024, 14, 7213–7219.
- 9
- 9aG.-H. Yang, Y. Li, X. Li, J.-P. Cheng, Chem. Sci. 2019, 10, 4322–4327;
- 9bZ. Huang, X. Huang, B. Li, C. Mou, S. Yang, B.-A. Song, Y. R. Chi, J. Am. Chem. Soc. 2016, 138, 7524–7527;
- 9cC. Hu, X. Tang, B. Zhang, Z. Zhang, W.-P. Deng, W. Zhang, ACS Catal. 2023, 13, 16300–16306;
- 9dZ.-C. Qi, Y. Li, J. Wang, L. Ma, G.-W. Wang, S.-D. Yang, ACS Catal. 2023, 13, 13301–13309;
- 9eY. Zheng, L. Guo, W. Zi, Org. Lett. 2018, 20, 7039–7043.
- 10
- 10aR.-Y. Zhu, L. Chen, X.-S. Hu, F. Zhou, J. Zhou, Chem. Sci. 2020, 11, 97–106;
- 10bG. Nishida, K. Noguchi, M. Hirano, K. Tanaka, Angew. Chem. Int. Ed. 2008, 47, 3410–3413; Angew. Chem. 2008, 120, 3458–3461;
- 10cY. Zhang, X. Liu, X. Feng, Abstr. Pap. Am. Chem. Soc. 2019, 258, 117980;
- 10dZ. Wang, T. Hayashi, Angew. Chem. Int. Ed. 2018, 57, 1702–1706; Angew. Chem. 2018, 130, 1718–1722;
- 10eJ. S. Harvey, S. J. Malcolmson, K. S. Dunne, S. J. Meek, A. L. Thompson, R. R. Schrock, A. H. Hoveyda, V. Gouverneur, Angew. Chem. Int. Ed. 2009, 48, 762–766; Angew. Chem. 2009, 121, 776–780.
- 11
- 11aM. Formica, T. Rogova, H. Shi, N. Sahara, B. Ferko, A. J. M. Farley, K. E. Christensen, F. Duarte, K. Yamazaki, D. J. Dixon, Nat. Chem. 2023, 15, 714–721;
- 11bK. C. Forbes, E. N. Jacobsen, Science 2022, 376, 1230–1236.
- 12
- 12aX.-L. Zhang, X. Qi, Y.-X. Wu, P. Liu, Y. He, Cell Rep. Phys. Sci. 2021, 2, 100594;
- 12bB. M. Trost, S. M. Spohr, A. B. Rolka, C. A. Kalnmals, J. Am. Chem. Soc. 2019, 141, 14098–14103.
- 13
- 13aW. Lv, Y. Chen, S. Wen, D. Ba, G. Cheng, J. Am. Chem. Soc. 2020, 142, 14864–14870;
- 13bW. Lv, S. Liu, Y. Chen, S. Wen, Y. Lan, G. Cheng, ACS Catal. 2020, 10, 10516–10522;
- 13cG. Cheng, P. Wang, J.-Q. Yu, Angew. Chem. Int. Ed. 2017, 56, 8183–8186; Angew. Chem. 2017, 129, 8295–8298;
- 13dW. Lv, J. Yu, B. Ge, S. Wen, G. Cheng, J. Org. Chem. 2018, 83, 12683–12693;
- 13eY. Chen, W. Lv, D. Ba, S. Wen, G. Cheng, J. Org. Chem. 2020, 85, 13280–13289;
- 13fY. Zhang, Y. Chen, Q. Tian, B. Wang, G. Cheng, J. Org. Chem. 2023, 88, 11793–11800;
- 13gW. Lv, S. Wen, J. Yu, G. Cheng, Org. Lett. 2018, 20, 4984–4987.
- 14
- 14aG. Cainelli, P. Galletti, D. Giacomini, Chem. Soc. Rev. 2009, 38, 990–1001;
- 14bA. Kumari, A. Jain, N. K. Rana, Tetrahedron 2024, 150, 100594;
- 14cM. Bartok, Chem. Rev. 2010, 110, 1663–1705.
- 15
- 15aN. Della Ca, M. Fontana, E. Motti, M. Catellani, Acc. Chem. Res. 2016, 49, 1389–1400;
- 15bZ. Chen, F. Zhang, Tetrahedron 2023, 134;
- 15cJ. Ye, M. Lautens, Nat. Chem. 2015, 7, 863–870;
- 15dJ. Wang, G. Dong, Chem. Rev. 2019, 119, 7478–7528;
- 15eR. Li, G. Dong, J. Am. Chem. Soc. 2020, 142, 17859–17875;
- 15fH.-G. Cheng, S. Chen, R. Chen, Q. Zhou, Angew. Chem. Int. Ed. 2019, 58, 5832–5844; Angew. Chem. 2019, 131, 5890–5902;
- 15gH.-G. Cheng, S. Jia, Q. Zhou, Acc. Chem. Res. 2023, 56, 573–591.
- 16
- 16aV. Sukowski, M. van Borselen, S. Mathew, M. A. Fernandez-Ibáñez, Angew. Chem. Int. Ed. 2022, 61, e202201750; Angew. Chem. 2022, 134, e202201750;
- 16bX. Liu, Y. Zhou, X. T. Qi, R. H. Li, P. Liu, G. B. Dong, Angew. Chem. Int. Ed. 2023, 62, e202310697; Angew. Chem. 2023, 135, e202310697;
- 16cS. Choi, G. B. Dong, J. Am. Chem. Soc. 2024, 146, 9512–9518;
- 16dA. J. Rago, R. Ye, X. Liu, G. B. Dong, Chem. Sci. 2024, 15, 1318–1323;
- 16eV. Sukowski, M. van Borselen, S. Mathew, B. de Bruin, M. A. Fernández-Ibáñez, Angew. Chem. Int. Ed. 2024, 63, e202317741; Angew. Chem. 2024, 136, e202317741;
- 16fF. Y. Wang, Y. X. Li, L. Jiao, J. Am. Chem. Soc. 2023, 145, 4871–4881;
- 16gM. Elsaid, R. Ge, C. Liu, D. Maiti, H. B. Ge, Angew. Chem. Int. Ed. 2023, 62, e202303110; Angew. Chem. 2023, 135, e202303110;
- 16hV. Botla, M. Fontana, A. Voronov, R. Maggi, E. Motti, G. Maestri, N. Della Ca, Angew. Chem. Int. Ed. 2023, 62, e202218928; Angew. Chem. 2023, 135, e202218928;
- 16iX. Liu, Y. Zhou, X. T. Qi, R. H. Li, P. Liu, G. B. Dong, Angew. Chem. Int. Ed. 2023, 62, e202310697; Angew. Chem. 2023, 135, e202310697;
- 16jY. X. Zheng, L. Jiao, Nat. Synth. 2022, 1, 180–187;
- 16kJ. Wang, C. Qin, J. P. Lumb, X. J. Luan, Chem. 2020, 6, 2097–2109.
- 17A. Rudolph, N. Rackelmann, M. Lautens, Angew. Chem. Int. Ed. 2007, 46, 1485–1488; Angew. Chem. 2007, 119, 1507–1510.
- 18L. L. Ding, X. W. Sui, Z. H. Gu, ACS Catal. 2018, 8, 5630–5635.
- 19X. M. Chen, L. Zhu, D. F. Chen, L. Z. Gong, Angew. Chem. Int. Ed. 2021, 60, 24844–24848; Angew. Chem. 2021, 133, 25048–25052.
- 20
- 20aH. Shi, A. N. Herron, Y. Shao, Q. Shao, J. Q. Yu, Nature 2018, 558, 581–585;
- 20bJ. J. Li, J. H. Zhao, H. C. Shen, K. V. Wu, X. Kuang, P. Wang, J. Q. Yu, Chem. 2023, 9, 1452–1463.
- 21R. H. Li, F. P. Liu, G. B. Dong, Org. Chem. Front. 2018, 5, 3108–3112.
- 22
- 22aZ. S. Liu, Y. Hua, Q. W. Gao, Y. Y. Ma, H. Tang, Y. Shang, H. G. Cheng, Q. H. Zhou, Nat. Catal. 2020, 3, 727–733;
- 22bY. Hua, Z. S. Liu, P. P. Xie, B. Ding, H. G. Cheng, X. Hong, Q. H. Zhou, Angew. Chem. Int. Ed. 2021, 60, 12824–12828; Angew. Chem. 2021, 133, 12934–12938;
- 22cZ. S. Liu, P. P. Xie, Y. Hua, C. G. Wu, Y. Y. Ma, J. W. Chen, H. G. Cheng, X. Hong, Q. H. Zhou, Chem. 2021, 7, 1917–1932;
- 22dQ. W. Gao, C. G. Wu, S. Deng, L. S. Li, Z. S. Liu, Y. Hua, J. X. Ye, C. Liu, H. G. Cheng, H. J. Cong, Y. C. Jiao, Q. H. Zhou, J. Am. Chem. Soc. 2021, 143, 7253–7260;
- 22eL. Zhou, H. G. Cheng, L. S. Li, K. Wu, J. Hou, C. K. Jiao, S. Deng, Z. R. Liu, J. Q. Yu, Q. H. Zhou, Nat. Chem. 2023, 15, 815–823;
- 22fZ. S. Liu, S. Deng, Q. W. Gao, Y. Hua, H. G. Cheng, X. T. Qi, Q. H. Zhou, ACS Catal. 2023, 13, 2968–2980;
- 22gJ. X. Ye, L. S. Li, Y. M. You, C. K. Jiao, Z. Y. Cui, Y. B. Zhang, S. H. Jia, H. J. Cong, S. S. Liu, H. G. Cheng, Q. H. Zhou, JACS Au 2023, 3, 384–390.
- 23Y. An, X. Y. Zhang, Y. N. Ding, Y. K. Li, X. Y. Liu, Y. M. Liang, Org. Lett. 2022, 24, 7294–7299.
- 24Deposition number 2335991 (for 4 x), contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 25J.-B. Pan, X.-G. Zhang, Y.-F. Shi, A.-C. Han, Y.-J. Chen, J. Ouyang, M.-L. Li, Q.-L. Zhou, Angew. Chem. Int. Ed. 2023, 62, e202300691; Angew. Chem. 2023, 135, e202300691.