Enantiopure Corral[4]BINOLs as Ultrastrong Receptors for Recognition and Differential Sensing of Steroids
Rong Fu
College of Chemistry, Nankai University, 94 Weijin Road, Tianjin, 300071 China
These authors contributed equally: Rong Fu, Dai-Yuan Li
Search for more papers by this authorDai-Yuan Li
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Collaborative Innovation Center of Chemical Science and Engineering, Nankai University, Tianjin, 300071 China
These authors contributed equally: Rong Fu, Dai-Yuan Li
Search for more papers by this authorJia-Hong Tian
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Collaborative Innovation Center of Chemical Science and Engineering, Nankai University, Tianjin, 300071 China
Search for more papers by this authorYi-Lin Lin
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Collaborative Innovation Center of Chemical Science and Engineering, Nankai University, Tianjin, 300071 China
Search for more papers by this authorQing-Yu Zhao
College of Chemistry, Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorWen-Li Li
College of Chemistry, Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorFang-Yuan Chen
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Collaborative Innovation Center of Chemical Science and Engineering, Nankai University, Tianjin, 300071 China
Search for more papers by this authorProf. Dr. Dong-Sheng Guo
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Collaborative Innovation Center of Chemical Science and Engineering, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Kang Cai
College of Chemistry, Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorRong Fu
College of Chemistry, Nankai University, 94 Weijin Road, Tianjin, 300071 China
These authors contributed equally: Rong Fu, Dai-Yuan Li
Search for more papers by this authorDai-Yuan Li
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Collaborative Innovation Center of Chemical Science and Engineering, Nankai University, Tianjin, 300071 China
These authors contributed equally: Rong Fu, Dai-Yuan Li
Search for more papers by this authorJia-Hong Tian
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Collaborative Innovation Center of Chemical Science and Engineering, Nankai University, Tianjin, 300071 China
Search for more papers by this authorYi-Lin Lin
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Collaborative Innovation Center of Chemical Science and Engineering, Nankai University, Tianjin, 300071 China
Search for more papers by this authorQing-Yu Zhao
College of Chemistry, Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorWen-Li Li
College of Chemistry, Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorFang-Yuan Chen
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Collaborative Innovation Center of Chemical Science and Engineering, Nankai University, Tianjin, 300071 China
Search for more papers by this authorProf. Dr. Dong-Sheng Guo
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, Collaborative Innovation Center of Chemical Science and Engineering, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Kang Cai
College of Chemistry, Nankai University, 94 Weijin Road, Tianjin, 300071 China
Search for more papers by this authorGraphical Abstract
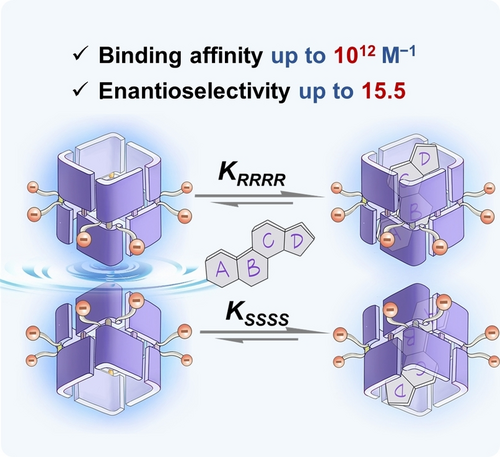
A pair of enantiomeric macrocycles, RRRR- and SSSS-C[4]BINOL, demonstrated exceptionally high recognition affinities (up to 1012 M−1) towards 16 important steroidal compounds in aqueous solutions, establishing them the most effective known steroid receptors. The two C[4]BINOL enantiomers were employed to compose a fluorescent sensor array which achieved discrimination and sensing of 16 structurally similar steroids in biofluids.
Abstract
The precise recognition and sensing of steroids, a type of vital biomolecules, hold immense practical value across various domains. In this study, we introduced corral[4]BINOLs (C[4]BINOLs), a pair of enantiomeric conjugated deep-cavity hosts, as novel synthetic receptors for binding steroids. Due to the strong hydrophobic effect of their deep nonpolar, chiral cavities, the two enantiomers of C[4]BINOLs demonstrated exceptionally high recognition affinities (up to 1012 M−1) for 16 important steroidal compounds as well as good enantioselectiviy (up to 15.5) in aqueous solutions, establishing them as the most potent known steroid receptors. Harnessing their ultrahigh affinity, remarkable enantioselectivity, and fluorescence emission properties, the two C[4]BINOL enantiomers were employed to compose a fluorescent sensor array which achieved discrimination and sensing of 16 structurally similar steroids at low concentrations.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202406233-sup-0001-misc_information.pdf3.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. M. Biros, J. J. Rebek, Chem. Soc. Rev. 2007, 36, 93–104;
- 1bL. Catti, R. Sumida, M. Yoshizawa, Coord. Chem. Rev. 2022, 460, 214460;
- 1cF.-Y. Chen, W.-C. Geng, K. Cai, D.-S. Guo, Chin. Chem. Lett. 2023, 109161;
- 1dJ. Chen, Y. Zhang, Y. Zhang, L. Zhao, L. Chen, Y. Chai, Z. Meng, X. Jia, Q. Meng, C. Li, Chin. Chem. Lett. 2021, 32, 3034–3038;
- 1eL. Cheng, K. Liu, Y. Duan, H. Duan, Y. Li, M. Gao, L. Cao, CCS Chem. 2020, 3, 2749–2763;
- 1fL. Escobar, P. Ballester, Chem. Rev. 2021, 121, 2445–2514;
- 1gY. Ferrand, M. P. Crump, A. P. Davis, Science 2007, 318, 619–622;
- 1hW. Gong, W. Wang, J. Dong, X. Pan, Y. Liu, H.-B. Yang, X. Fu, Y. Cui, CCS Chem. 2023, 0, 1–24;
- 1iT. Guinovart, D. Hernández-Alonso, L. Adriaenssens, P. Blondeau, M. Martínez-Belmonte, F. X. Rius, F. J. Andrade, P. Ballester, Angew. Chem. Int. Ed. 2016, 55, 2435–2440;
- 1jX.-Y. Hu, R. Fu, D.-S. Guo, Acc. Mater. Res. 2023, 4, 925–938;
- 1kC. Ke, H. Destecroix, M. P. Crump, A. P. Davis, Nat. Chem. 2012, 4, 718–723;
- 1lT. Li, X. Zhu, G. Ouyang, M. Liu, Mater. Chem. Front. 2023, 7, 3879–3903;
- 1mZ. Li, Y.-W. Yang, Acc. Mater. Res. 2021, 2, 292–305;
- 1nW. Liu, S. K. Samanta, B. D. Smith, L. Isaacs, Chem. Soc. Rev. 2017, 46, 2391–2403;
- 1oY. H. Liu, Y. M. Zhang, H. J. Yu, Y. Liu, Angew. Chem. Int. Ed. 2021, 60, 3870–3880;
- 1pD. Ma, G. Hettiarachchi, D. Nguyen, B. Zhang, J. B. Wittenberg, P. Y. Zavalij, V. Briken, L. Isaacs, Nat. Chem. 2012, 4, 503–510;
- 1qL. Pu, Angew. Chem. Int. Ed. 2020, 59, 21814–21828;
- 1rM. Quan, X. Y. Pang, W. Jiang, Angew. Chem. Int. Ed. 2022, 61, e202201258;
- 1sA. J. Selinger, N. A. Cavallin, A. Yanai, I. Birol, F. Hof, Angew. Chem. Int. Ed. 2022, 61, e202113235;
- 1tR. Wang, W.-B. Li, J.-Y. Deng, H. Han, F.-Y. Chen, D.-Y. Li, L.-B. Jing, Z. Song, R. Fu, D.-S. Guo, K. Cai, Angew. Chem. Int. Ed. 2024, 63, e202317402;
- 1uX. Yang, X. Gao, Y.-X. Zheng, H. Kuang, C.-F. Chen, M. Liu, P. Duan, Z. Tang, CCS Chem. 2023, 1–30;
- 1vM. Yoshizawa, L. Catti, Acc. Chem. Res. 2019, 52, 2392–2404;
- 1wY.-X. Yue, Y.-L. Lin, M.-M. Chen, H.-W. Tian, R. Ma, Z.-H. Wang, F.-Y. Chen, Y.-C. Pan, D.-S. Guo, Sci. China Chem. 2023, DOI: 10.1007/s11426-11023-11857-11422;
- 1xH. Han, R. Fu, R. Wang, C. Tang, M.-M. He, J.-Y. Deng, D.-S. Guo, J. F. Stoddart, K. Cai, J. Am. Chem. Soc. 2022, 144, 20351–20362;
- 1yZ. Y. Zhang, C. Li, Acc. Chem. Res. 2022, 55, 916–929;
- 1zY. Zhou, H. Li, Y.-W. Yang, Chin. Chem. Lett. 2015, 26, 825–828.
- 2
- 2aD. N. Kirk, B. A. Marples, in Steroid Analysis (Eds.: H. L. J. Makin, D. B. Gower, D. N. Kirk), Springer Netherlands, Dordrecht 1995, pp. 1–24;
10.1007/978-94-017-3078-5_1 Google Scholar
- 2bT. Friščić, R. W. Lancaster, L. Fábián, P. G. Karamertzanis, Proc. Natl. Acad. Sci. USA 2010, 107, 13216–13221.
- 3A. W. Norman, M. T. Mizwicki, D. P. G. Norman, Nat. Rev. Drug Discovery 2004, 3, 27–41.
- 4
- 4aF. Faassen, J. Kelder, J. Lenders, R. Onderwater, H. Vromans, Pharm. Res. 2003, 20, 177–186;
- 4bW. Rosner, D. J. Hryb, M. S. Khan, A. M. Nakhla, N. A. Romas, Steroids 1998, 63, 278–281.
- 5R. Lösel, M. Wehling, Nat. Rev. Mol. Cell Biol. 2003, 4, 46–55.
- 6
- 6aJ. C. Wagner, Sports Med. 1991, 12, 250–265;
- 6bIntechOpen, Rijeka 2012.
- 7Y. Liu, Y. Song, Y. Chen, X.-Q. Li, F. Ding, R.-Q. Zhong, Chem. Eur. J. 2004, 10, 3685–3696.
- 8P. Jansook, T. Loftsson, Int. J. Pharm. 2008, 363, 217–219.
- 9
- 9aA. Bom, M. Bradley, K. Cameron, J. K. Clark, J. van Egmond, H. Feilden, E. J. MacLean, A. W. Muir, R. Palin, D. C. Rees, M.-Q. Zhang, Angew. Chem. Int. Ed. 2002, 41, 265;
- 9bÉ. Fenyvesi, I. Puskás, L. Szente, Environ. Chem. Lett. 2018, 17, 375–391;
- 9cY.-M. Zhang, X. Xu, Q. Yu, Y.-H. Liu, Y.-H. Zhang, L.-X. Chen, Y. Liu, J. Med. Chem. 2017, 60, 3266–3274.
- 10A. S. Akha, J. Rosa, J. S. Jahr, A. Li, K. Kiai, Anesthesiol. Clin. 2010, 28, 691–708.
- 11
- 11aC. L. D. Gibb, B. C. Gibb, J. Am. Chem. Soc. 2004, 126, 11408–11409;
- 11bA. D. Gill, L. Perez, I. N. Q. Salinas, S. R. Byers, Y. Liu, B. L. Hickey, W. Zhong, R. J. Hooley, Chem. Eur. J. 2019, 25, 1740–1745.
- 12
- 12aA. I. Lazar, F. Biedermann, K. R. Mustafina, K. I. Assaf, A. Hennig, W. M. Nau, J. Am. Chem. Soc. 2016, 138, 13022–13029;
- 12bD. Ma, B. Zhang, U. Hoffmann, M. G. Sundrup, M. Eikermann, L. Isaacs, Angew. Chem. Int. Ed. 2012, 51, 11358–11362.
- 13
- 13aG. Li, T. K. Ronson, R. Lavendomme, Z. H. Huang, C. Fuertes-Espinosa, D. W. Zhang, J. R. Nitschke, Chem 2023, 9, 1549–1561;
- 13bF. J. Rizzuto, J. P. Carpenter, J. R. Nitschke, J. Am. Chem. Soc. 2019, 141, 9087–9095;
- 13cM. Yamashina, T. Tsutsui, Y. Sei, M. Akita, M. Yoshizawa, Sci. Adv. 2019, 5, eaav3179.
- 14
- 14aC. Li, Y. Wei, S. Zhang, W. Tan, Environ. Chem. Lett. 2020, 18, 543–559;
- 14bS. Sarkar, P. Ballester, M. Spektor, E. A. Kataev, Angew. Chem. Int. Ed. 2023, 62, e202214705.
- 15R. Fu, Q.-Y. Zhao, H. Han, W.-L. Li, F.-Y. Chen, C. Tang, W. Zhang, S.-D. Guo, D.-Y. Li, W.-C. Geng, D.-S. Guo, K. Cai, Angew. Chem. Int. Ed. 2023, 62, e202315990.
- 16While C[4]BINOLs feature several carboxylic acid groups, the alterations in pH within the aqueous solution during our fluorometric titrations are negligible due to the low concentrations of both the hosts and guests employed. This has been substantiated through pH measurements conducted before and after the titrations. Furthermore, our previous research (see Ref 16) has indicated that, whether in pure water or buffered solutions, C[4]BINOLs exhibit comparable binding affinities towards neutral guests.
- 17P. Thomas, Cells 2022, 11, 1785.
- 18
- 18aJ. W. Barnett, M. R. Sullivan, J. A. Long, D. Tang, T. Nguyen, D. Ben-Amotz, B. C. Gibb, H. S. Ashbaugh, Nat. Chem. 2020, 12, 589–594;
- 18bF. Biedermann, W. M. Nau, H. J. Schneider, Angew. Chem. Int. Ed. 2014, 53, 11158–11171;
- 18cF. Biedermann, H.-J. Schneider, Chem. Rev. 2016, 116, 5216–5300.
- 19
- 19aX. N. Han, P. F. Li, Y. Han, C. F. Chen, Angew. Chem. Int. Ed. 2022, 61, e202202527;
- 19bH. Chai, Z. Chen, S.-H. Wang, M. Quan, L.-P. Yang, H. Ke, W. Jiang, CCS Chem. 2020, 2, 440–452;
- 19cM. V. Rekharsky, Y. Inoue, Chem. Rev. 1998, 98, 1875–1918.
- 20
- 20aK. S. Cameron, J. K. Clark, A. Cooper, L. Fielding, R. Palin, S. J. Rutherford, M. Q. Zhang, Org. Lett. 2002, 4, 3403–3406;
- 20bP. Wallimann, T. Marti, A. Fürer, F. Diederich, Chem. Rev. 1997, 97, 1567–1608.
- 21J. R. Sempionatto, J. A. Lasalde-Ramírez, K. Mahato, J. Wang, W. Gao, Nat. Chem. Rev. 2022, 6, 899–915.
- 22M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, Williams, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, D. J. Fox, Gaussian, Inc., Wallingford, CT 2016.
- 23
- 23aT. Lu, F. Chen, J. Comput. Chem. 2012, 33, 580–592;
- 23bW. Humphrey, A. Dalke, K. Schulten, J. Mol. Graphics 1996, 14, 33–38.
- 24
- 24aR. Kumar, A. Sharma, H. Singh, P. Suating, H. S. Kim, K. Sunwoo, I. Shim, B. C. Gibb, J. S. Kim, Chem. Rev. 2019, 119, 9657–9721;
- 24bV. Francisco, A. Piñeiro, W. M. Nau, L. García-Río, Chem. Eur. J. 2013, 19, 17809–17820;
- 24cS. C. Hong, D. P. Murale, M. Lee, S. M. Lee, J. S. Park, J. S. Lee, Angew. Chem. Int. Ed. 2017, 56, 14642–14647.
- 25
- 25aA. J. Selinger, F. Hof, Angew. Chem. Int. Ed. 2023, 62, e202312407;
- 25bJ.-H. Tian, X.-Y. Hu, Z.-Y. Hu, H.-W. Tian, J.-J. Li, Y.-C. Pan, H.-B. Li, D.-S. Guo, Nat. Commun. 2022, 13, 4293.
- 26
- 26aR. Leiva, T. Bouchard, H. Boehringer, S. Abulla, R. Ecochard, Steroids 2015, 101, 125–129;
- 26bB. Nagy, J. Szekeres-Bartho, G. L. Kovacs, E. Sulyok, B. Farkas, A. Varnagy, V. Vertes, K. Kovacs, J. Bodis, Int. J. Mol. Sci. 2021, 22, 11039.
- 27E. Radwanska, J. Frankenberg, E. I. Allen, Fertil. Steril. 1978, 30, 398–402.