Cooperative Photoredox and Cobalt-Catalyzed Acceptorless Dehydrogenative Functionalization of Cyclopropylamides towards Allylic N,O-Acyl-acetal Derivatives
Haohao Huang
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education College of Chemistry & Materials Science, Northwest University, Xi'an, 710127 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xinjun Luan
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education College of Chemistry & Materials Science, Northwest University, Xi'an, 710127 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhijun Zuo
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education College of Chemistry & Materials Science, Northwest University, Xi'an, 710127 China
Search for more papers by this authorHaohao Huang
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education College of Chemistry & Materials Science, Northwest University, Xi'an, 710127 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xinjun Luan
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education College of Chemistry & Materials Science, Northwest University, Xi'an, 710127 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhijun Zuo
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education College of Chemistry & Materials Science, Northwest University, Xi'an, 710127 China
Search for more papers by this authorGraphical Abstract
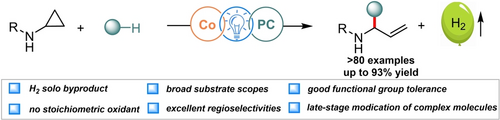
We report a novel chemoselective dehydrogenative functionalization of cyclopropylamides enabled by cooperative photoredox and cobalt catalysis for forging the allylic N,O-acyl-acetal scaffolds in an environmental-friendly and atom-economic fashion. The starting materials were readily available and the transformation proceeded under extremely mild conditions with good functional group tolerance and broad scopes.
Abstract
We disclose herein a novel photoredox and cobalt co-catalyzed ring-opening/acceptorless dehydrogenative functionalization of mono-donor cyclopropanes. This sustainable and atom-economic approach allows the rapid assembly of a wide range of allylic N,O-acyl-acetal derivatives. The starting materials are readily available and the reaction features mild conditions, broad substrate scope, and excellent functional group compatibility. The optimized conditions accommodate assorted cycloalkylamides and primary, secondary, and tertiary alcohols, with applications in late-stage functionalization of pharmaceutically relevant compounds, stimulating further utility in medicinal chemistry. Moreover, selective nucleophilic substitutions with various carbon nucleophiles were achieved in a one-pot fashion, offering a reliable avenue to access some cyclic and acyclic derivatives.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202401579-sup-0001-misc_information.pdf15.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. B. Simth, I. G. Safonov, R. M. Corbett, J. Am. Chem. Soc. 2001, 123, 12426;
- 1bR. H. Cichewicz, F. A. Valeriote, P. Crews, Org. Lett. 2004, 6, 1951;
- 1cR. A. Mosey, P. E. Floreancig, Nat. Prod. Rep. 2012, 29, 980;
- 1dM. A. Tammam, A. El-Demerdash, Curr. Opin. Biotechnol. 2023, 6, 100145.
- 2
- 2aC. Vanier, A. Wagner, C. Mioskowski, Chem. Eur. J. 2001, 7, 2318;
10.1002/1521-3765(20010601)7:11<2318::AID-CHEM23180>3.0.CO;2-W CAS PubMed Web of Science® Google Scholar
- 2bH. Kim, Y. H. Rhee, J. Am. Chem. Soc. 2012, 134, 4011;
- 2cM. Li, B. Luo, Q. Liu, Y. Hu, A. Ganesan, P. Huang, S. Wen, Org. Lett. 2014, 16, 10;
- 2dJ. Halli, K. Hofman, T. Beisel, G. Manolikakes, Eur. J. Org. Chem. 2015, 4624;
- 2eJ. Ranjith, P. R. Krishna, Tetrahedron Lett. 2019, 60, 1437.
- 3
- 3aG. N. Schrauzer, Acc. Chem. Res. 1968, 1, 97;
- 3bM. Giedyk, K. Goliszewska, D. Gryko, Chem. Soc. Rev. 2015, 44, 3391.
- 4
- 4aV. Artero, M. Chavarot-Kerlidou, M. Fontecave, Angew. Chem. 2011, 123, 7376;
10.1002/ange.201007987 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 7238;
- 4bL. Wu, B. Chen, Z. Li, C.-H. Tung, Acc. Chem. Res. 2014, 47, 2177.
- 5
- 5aQ. Liu, L. Wu, Natl. Sci. Rev. 2017, 4, 359;
- 5bB. Chen, L. Wu, C.-H. Tung, Acc. Chem. Res. 2018, 51, 2512;
- 5cK. C. Cartwright, A. M. Davies, J. A. Tunge, Eur. J. Org. Chem. 2020, 1245.
- 6
- 6aQ. Meng, J. Zhong, Q. Liu, X. Gao, H. Zhang, T. Lei, Z. Li, K. Feng, B. Chen, C. Tung, L. Wu, J. Am. Chem. Soc. 2013, 135, 19052;
- 6bJ. Zhong, Q. Meng, B. Liu, X. Li, X. Gao, T. Lei, C. Wu, Z. Li, C. Tung, L. Wu, Org. Lett. 2014, 16, 1988;
- 6cG. Zhang, C. Liu, H. Yi, Q. Meng, C. Bian, H. Chen, J. Jian, L. Wu, A. Lei, J. Am. Chem. Soc. 2015, 137, 9273;
- 6dH. Cao, H. Jiang, H. Feng, J. M. C. Kwan, X. Liu, J. Wu, J. Am. Chem. Soc. 2018, 140, 16360;
- 6eK. C. Cartwright, J. A. Tunge, ACS Catal. 2018, 8, 11801;
- 6fS. U. Dighe, F. Juliá, A. Luridiana, J. J. Douglas, D. A. Leonori, Nature 2020, 584, 75.
- 7For a book, see:
- 7aO. G. Kulinkovich, Cyclopropanes in Organic Synthesis, Wiley-VCH, Weinheim 2015; For selected reviews, see:
10.1002/9781118978429 Google Scholar
- 7bA. de Meijere, Angew. Chem. 1979, 91, 867; Angew. Chem. Int. Ed. Engl. 1979, 18, 809;
- 7cH. N. C. Wong, M. Y. Hon, C. W. Tse, Y. C. Yip, J. Tanko, T. Hudlicky, Chem. Rev. 1989, 89, 165;
- 7dM. Rubin, M. Rubina, V. Gevorgyan, Chem. Rev. 2007, 107, 3117;
- 7eZ. Zhu, Y. Wei, M. Shi, Chem. Soc. Rev. 2011, 40, 5534;
- 7fD. Y.-K. Chen, R. H. Pouwer, J.-A. Richard, Chem. Soc. Rev. 2012, 41, 4631;
- 7gC. Ebner, E. M. Carreira, Chem. Rev. 2017, 117, 11651;
- 7hL. Dian, I. Marck, Chem. Rev. 2018, 118, 8415.
- 8
- 8aC. A. Carson, M. A. Kerr, Chem. Soc. Rev. 2009, 38, 3501;
- 8bG. Fumagali, S. Stanton, J. F. Bower, Chem. Rev. 2017, 117, 9404;
- 8cA. S. Harmata, B. J. Roldan, C. R. J. Stephenson, Angew. Chem. 2023, 135, e202213003;
10.1002/ange.202213003 Google ScholarAngew. Chem. Int. Ed. 2023, 62, e202213003.
- 9
- 9aA. B. Leduc, M. A. Kerr, Angew. Chem. 2008, 120, 8603;
10.1002/ange.200803257 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7945;
- 9bA. T. Parsons, J. S. Johnson, J. Am. Chem. Soc. 2009, 131, 3122;
- 9cF. de Nanteuil, J. Waser, Angew. Chem. 2011, 123, 12281;
10.1002/ange.201106255 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 12075;
- 9dY. Zhou, L. Wang, J. Li, X. Sun, Y. Tang, J. Am. Chem. Soc. 2012, 134, 9066;
- 9eS. Das, C. G. Daniliuc, A. Studer, Angew. Chem. 2017, 129, 11712; Angew. Chem. Int. Ed. 2017, 56, 11554.
- 10
- 10aT. Parsons, A. M. J. Campbell, J. S. Johnson, Org. Lett. 2008, 10, 2541;
- 10bR. Tombe, T. Kurahashi, S. Matsubara, Org. Lett. 2013, 15, 1791;
- 10cB. M. Trost, Z. Zuo, Angew. Chem. 2021, 133, 5870;
10.1002/ange.202016439 Google ScholarAngew. Chem. Int. Ed. 2021, 60, 5806.
- 11
- 11aS. Kolb, M. Petzold, F. Brandt, P. G. Jones, C. R. Jacob, D. B. Werz, Angew. Chem. 2021, 133, 16064; Angew. Chem. Int. Ed. 2021, 60, 15928;
- 11bS. Kolb, N. L. Ahlburg, D. B. Werz, Org. Lett. 2021, 23, 5549.
- 12
- 12aZ. Lu, M. Shen, T. P. Yoon, J. Am. Chem. Soc. 2011, 133, 1162;
- 12bT. V. T. Nguyen, A. Bossonnet, M. D. Wodrich, J. Waser, J. Am. Chem. Soc. 2023, 145, 25411.
- 13
- 13aZ. Lu, J. D. Parrish, T. P. Yoon, Tetrahedron 2014, 70, 4270;
- 13bS. Yang, L. Wang, H. Zhang, C. Liu, L. Zhang, X. Wang, G. Zhang, Y. Li, Q. Zhang, ACS Catal. 2019, 9, 716;
- 13cL. Ge, C. Zhang, C. Pan, X. Wang, D. Liu, Z. Li, P. Shen, L. Tian, C. Feng, Nat. Commun. 2019, 10, 4367;
- 13dP. Peng, X. Yan, K. Zhang, Z. Liu, L. Zeng, Y. Chen, H. Zhang, A. Lei, Nat. Commun. 2021, 13, 3075;
- 13eZ. Zuo, C. G. Daniliuc, A. Studer, Angew. Chem. 2021, 133, 25456; Angew. Chem. Int. Ed. 2021, 60, 25252;
- 13fY. Xu, H. Gao, C. Pan, Y. Shi, C. Zhang, G. Huang, C. Feng, Angew. Chem. 2023, 135, e202310671; Angew. Chem. Int. Ed. 2023, 62, e202310671.
- 14
- 14aM. Murai, A. Nishiyama, N. Nishinaka, H. Morita, K. Takai, Chem. Commun. 2017, 53, 9281;
- 14bD. Wang, X. Xue, K. N. Houk, Z. Shi, Angew. Chem. 2018, 130, 17103; Angew. Chem. Int. Ed. 2018, 57, 16861;
- 14cE. Richmond, J. Yi, V. D. Vuković, F. Sajadi, C. N. Rowley, J. Moran, Chem. Sci. 2018, 9, 6411;
- 14dA. Roy, V. Bonetti, G. Wang, Q. Wu, H. F. T. Klare, M. Oestreich, Org. Lett. 2020, 22, 1213;
- 14eM. H. Gieuw, S. Chen, Z. Ke, K. N. Houk, Y.-Y. Yeung, Chem. Sci. 2020, 11, 9426.
- 15
- 15aJ. G. M. Morton, M. A. Dureen, D. W. Stephan, Chem. Commun. 2010, 46, 8947;
- 15bZ. Zhang, Z. Liu, R. Guo, Y. Zhao, X. Li, X. Wang, Angew. Chem. 2017, 129, 4086; Angew. Chem. Int. Ed. 2017, 56, 4028;
- 15cZ. Zhang, J. Ren M Zhang, X. Xu, X. Wang, Chin. J. Chem. 2021, 39, 1641.
- 16
- 16aS. Maity, M. Zhu, R. S. Shinabery, N. Zheng, Angew. Chem. 2012, 124, 226; Angew. Chem. Int. Ed. 2012, 51, 222;
- 16bT. H. Nguyen, S. A. Morris, N. Zheng, Adv. Synth. Catal. 2014, 356, 2831;
- 16cQ. Wang, N. Zhang, Org. Lett. 2019, 21, 9999.
- 17
- 17aB. Muriel, A. Gagnebin, J. Waser, Chem. Sci. 2019, 10, 10716;
- 17bM. Wang, J. Waser, Angew. Chem. 2020, 132, 16562; Angew. Chem. Int. Ed. 2020, 59, 16420;
- 17cM. Wang, S. Jeon, J. Waser, Org. Lett. 2020, 22, 9123;
- 17dM. Wang, T. V. T. Nguyen, J. Waser, J. Am. Chem. Soc. 2021, 143, 11969.
- 18
- 18aC. Madelaine, Y. Six, O. Buriez, Angew. Chem. 2007, 119, 8192;
10.1002/ange.200702903 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8046;
- 18bY. Cai, J. Wang, Y. Zhang, Z. Li, D. Hu, N. Zheng, H. Chen, J. Am. Chem. Soc. 2017, 139, 12259;
- 18cD. Staveness, T. M. Sodano, K. Li, E. A. Burnham, K. D. Jackson, C. R. J. Stephenson, Chem 2019, 5, 215;
- 18dY. Yin, Y. Li, T. P. Gonca̧lves, Q. Zhan, G. Wang, X. Zhao, B. Qiao, W. Huang, Z. Jiang, J. Am. Chem. Soc. 2020, 142, 19451;
- 18eD. Uraguchi, Y. Kimura, F. Ueoka, T. Ooi, J. Am. Chem. Soc. 2020, 142, 19462;
- 18fZ. Liu, S. Wu, Y. Chen, ACS Catal. 2021, 11, 10565.
- 19
- 19aO. O. Sokolova, J. F. Bower, Chem. Rev. 2021, 121, 80;
- 19bM. Wang, T. V. T. Nguyen, J. Waser, Chem. Soc. Rev. 2022, 51, 7344.
- 20The screening of other kinds of aminocyclopropanes see SI.
- 21
- 21aC. Dai, J. Genovino, B. M. Bechle, M. S. Corbett, C. W. Huh, C. R. Rose, J. Sun, J. S. Warmus, D. C. Blakemore, Org. Lett. 2017, 19, 1064;
- 21bM. Wang, J. Waser, Angew. Chem. 2019, 131, 14018; Angew. Chem. Int. Ed. 2019, 58, 13880.
- 22K. Yamashita, R. Hirokawa, M. Ichikawa, T. Hisanaga, Y. Nagao, R. Takita, K. Watanabe, Y. Kawato, Y. Hamashima, J. Am. Chem. Soc. 2022, 144, 3913.
- 23M. L. Grimm, N. K. Suleman, A. N. Hancock, J. N. Spencer, T. Dudding, R. Rowshanpour, N. Castagnoli Jr, J. M. Tanko, J. Am. Chem. Soc. 2020, 142, 2640.
- 24
- 24aM. A. Cismesia, T. P. Yoon, Chem. Sci. 2015, 6, 5426;
- 24bL. Buzzetti, G. E. M. Crisenza, P. Melchiorre, Angew. Chem. 2018, 131, 3768;
10.1002/ange.201809984 Google ScholarAngew. Chem. Int. Ed. 2018, 58, 3730.
- 25
- 25aM. Zhao, WO2007038452 A1, 2007;
- 25bY. Seiji, S. Ryota, K. Tomoaki, S. Daisuke, H. Mitsuru, F. Hiroki, K. Kazuo, I. Hirofumi, K. Takanori, WO2007105753 A1, 2007;
- 25cG. A. Molander, S. A. McKee, Org. Lett. 2011, 13, 4684;
- 25dF. Muth, M. Günther, S. M. Bauer, E. Döring, S. Fischer, J. Maier, P. Drückes, J. Köppler, J. Trapper, U. Rothbauer, P. Koch, S. A. Laufer, J. Med. Chem. 2015, 58, 443;
- 25eG. Choi, Q. Zhu, D. Miller, C. J. Gu, R. R. Knowles, Nature 2016, 539, 268;
- 25fH. Kondo, K. Itami, J. Yamaguchi, Chem. Sci. 2017, 8, 3799;
- 25gY. Wang, J. Bai, Y. Yang, W. Zhao, Y. Liang, D. Wang, Chem. Sci. 2021, 12, 3599;
- 25hT. M. Monos, R. C. Mactee, C. R. J. Stephenson, Science 2018, 361, 1369;
- 25iX. Sun, J. Chen, T. Ritter, Nat. Chem. 2018, 10, 1229;
- 25jM. Dawidowskia, M. Króla, B. Szulczyka, A. Chodkowskia, P. Podsadnia, P. Konopelski, M. Ufnal, P. Szuberski, M. Z. Wróbel, Y. Zhang, A. El Harchi, J. C. Hancox, D. Jarkovska, E. Mistrova, J. Sviglerova, M. Štengl, G. M. Popowicz, J. Turɬo, Bioorg. Chem. 2020, 98, 103717;
- 25kK. Yamashita, R. Hirokawa, M. Ichikawa, T. Hisanaga, Y. Nagao, J. Am. Chem. Soc. 2022, 144, 3913.