Controllable Skeletal and Peripheral Editing of Pyrroles with Vinylcarbenes
Yong Yang
Department of Chemistry, Northeast Normal University, Changchun, 130024 China
These authors contributed equally to this work.
Search for more papers by this authorQingmin Song
Department of Chemistry, Northeast Normal University, Changchun, 130024 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Paramasivam Sivaguru
Department of Chemistry, Northeast Normal University, Changchun, 130024 China
Search for more papers by this authorCorresponding Author
Dr. Zhaohong Liu
Department of Chemistry, Northeast Normal University, Changchun, 130024 China
Search for more papers by this authorDan Shi
Department of Chemistry, Northeast Normal University, Changchun, 130024 China
Search for more papers by this authorTian Tian
Department of Chemistry, Northeast Normal University, Changchun, 130024 China
Search for more papers by this authorGraham de Ruiter
Schulich Faculty of Chemistry, Technion—Israel Institute of Technol-ogy Technion City, 3200008 Haifa, Israel
Search for more papers by this authorCorresponding Author
Prof. Xihe Bi
Department of Chemistry, Northeast Normal University, Changchun, 130024 China
State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorYong Yang
Department of Chemistry, Northeast Normal University, Changchun, 130024 China
These authors contributed equally to this work.
Search for more papers by this authorQingmin Song
Department of Chemistry, Northeast Normal University, Changchun, 130024 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Paramasivam Sivaguru
Department of Chemistry, Northeast Normal University, Changchun, 130024 China
Search for more papers by this authorCorresponding Author
Dr. Zhaohong Liu
Department of Chemistry, Northeast Normal University, Changchun, 130024 China
Search for more papers by this authorDan Shi
Department of Chemistry, Northeast Normal University, Changchun, 130024 China
Search for more papers by this authorTian Tian
Department of Chemistry, Northeast Normal University, Changchun, 130024 China
Search for more papers by this authorGraham de Ruiter
Schulich Faculty of Chemistry, Technion—Israel Institute of Technol-ogy Technion City, 3200008 Haifa, Israel
Search for more papers by this authorCorresponding Author
Prof. Xihe Bi
Department of Chemistry, Northeast Normal University, Changchun, 130024 China
State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorGraphical Abstract
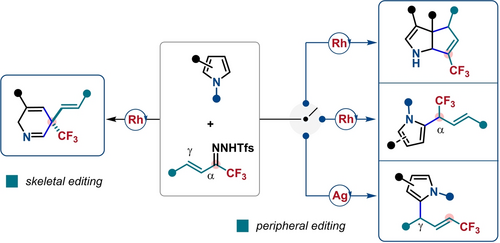
A highly controllable molecular editing strategy of pyrroles was achieved by using trifluoromethyl vinyl N-triftosylhydrazones as vinylcarbene precursors, which led to the first dearomative single-atom skeletal editing of pyrroles through carbon-atom insertion, as well as three types of peripheral editing reactions: α- or γ-selective C−H insertion, and [3+2] cycloaddition.
Abstract
The skeletal editing of azaarenes through insertion, deletion, or swapping of single atoms has recently gained considerable momentum in chemical synthesis. Here, we describe a practical skeletal editing strategy using vinylcarbenes in situ generated from trifluoromethyl vinyl N-triftosylhydrazones, leading to the first dearomative skeletal editing of pyrroles through carbon-atom insertion. Furthermore, depending on the used catalyst and substrate, three types of peripheral editing reactions of pyrroles are also disclosed: α- or γ-selective C−H insertion, and [3+2] cycloaddition. These controllable molecular editing reactions provide a powerful platform for accessing medicinally relevant CF3-containing N-heterocyclic frameworks, such as 2,5-dihydropyridines, piperidines, azabicyclo[3.3.0]octadienes, and allylated pyrroles from readily available pyrroles. Mechanistic insights from experiments and density functional theory (DFT) calculations shed light on the origin of substrate- or catalyst-controlled chemo- and regioselectivity as well as the reaction mechanism.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data supporting the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202401359-sup-0001-misc_information.pdf10.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Woo, A. H. Christian, S. A. Burgess, Y. Jiang, U. F. Mansoor, M. D. Levin, Science 2022, 376, 527–532;
- 1bJ. Woo, C. Stein, A. H. Christian, M. D. Levin, Nature 2023, 623, 77–82;
- 1cT. J. Pearson, R. Shimazumi, J. L. Driscoll, B. D. Dherange, D.-I. Park, M. D. Levin, Science 2023, 381, 1474–1479;
- 1dS. H. Kennedy, B. D. Dherange, K. J. Berger, M. D. Levin, Nature 2021, 593, 223–227;
- 1eJ. B. Roque, Y. Kuroda, L. T. Göttemann, R. Sarpong, Nature 2018, 564, 244–248;
- 1fJ. Jurczyk, M. C. Lux, D. Adpressa, S. F. Kim, Y. Lam, C. S. Yeung, R. Sarpong, Science 2021, 373, 1004–1012;
- 1gJ. C. Reisenbauer, O. Green, A. Franchino, P. Finkelstein, B. Morandi, Science 2022, 377, 1104–1109;
- 1hH. Wang, H. Shao, A. Das, S. Dutta, H. T. Chan, C. Daniliuc, K. N. Houk, F. Glorius, Science 2023, 381, 75–81;
- 1iH. Lyu, I. Kevlishvili, X. Yu, P. Liu, G. Dong, Science 2021, 372, 175–182.
- 2
- 2aL. Guillemard, N. Kaplaneris, L. Ackermann, M. J. Johansson, Nat. Chem. Rev. 2021, 5, 522–545;
- 2bH. M. L. Davies, K. Liao, Nat. Chem. Rev. 2019, 3, 347–360;
- 2cJ. Wencel-Delord, F. Glorius, Nat. Chem. 2013, 5, 369–375.
- 3J. Jurczyk, J. Woo, S. F. Kim, B. D. Dherange, R. Sarpong, M. D. Levin, Nat. Synth. 2022, 1, 352–364.
- 4M. Peplow, Nature. 2023, 618, 21–24.
- 5A. M. Szpilman, E. M. Carreira, Angew. Chem. Int. Ed. 2010, 49, 9592–9628.
- 6Z. Liu, P. Sivaguru, Y. Ning, Y. Wu, X. Bi, Chem. A Eur. J. 2023, 29, e202301227.
- 7B. W. Joynson, L. T. Ball, Helv. Chim. Acta. 2023, 106, e202200182.
- 8
- 8aM. Mortén, M. Martin Hennum, T. Bonge-Hansen, Beilstein J. Org. Chem. 2015, 11, 1944–1949;
- 8bG. L. Bartholomew, F. Carpaneto, R. Sarpong, J. Am. Chem. Soc. 2022, 144, 22309–22315;
- 8cE. E. Hyland, P. Q. Kelly, A. M. McKillop, B. D. Dherange, M. D. Levin, J. Am. Chem. Soc. 2022, 144, 19258–19264;
- 8dJ. C. Reisenbauer, A. S. K. Paschke, J. Krizic, B. B. Botlik, P. Finkelstein, B. Morandi, Org. Lett. 2023, 25, 8419–8423;
- 8eC.-M. Chen, Y.-N. Yang, Y.-Z. Kong, B.-H. Zhu, P.-C. Qian, B. Zhou, L.-W. Ye, Commun. Chem. 2023, 6, 194;
- 8fY. Zhou, F. Chen, Z. Li, J. Dong, J. Li, B. Zhang, Q. Song, Sci. China Chem. 2023, 66, 1975–1981.
- 9C. T. Walsh, S. Garneau-Tsodikova, A. R. Howard-Jones, Nat. Prod. Rep. 2006, 23, 517–531.
- 10
- 10aM. Baumann, I. R. Baxendale, S. V. Ley, N. Nikbin, Beilstein J. Org. Chem. 2011, 7, 442–495;
- 10bM. K. Hunjan, S. Panday, A. Gupta, J. Bhaumik, P. Das, J. K. Laha, Chem. Rec. 2021, 21, 715–780.
- 11J. Liu, H. Li, A. Spannenberg, R. Franke, R. Jackstell, M. Beller, Angew. Chem. Int. Ed. 2016, 55, 13544–13548.
- 12T. V. Vernitskaya, O. N. Efimov, Russ. Chem. Rev. 1997, 66, 443–457.
10.1070/RC1997v066n05ABEH000261 Google Scholar
- 13E. Vitaku, D. T. Smith, J. T. Njardarson, J. Med. Chem. 2014, 57, 10257–10274.
- 14G. L. Ciamician, M. Dennstedt, Ber. Dtsch. Chem. Ges. 1881, 14, 1153–1163.
10.1002/cber.188101401240 Google Scholar
- 15D. Ma, B. S. Martin, K. S. Gallagher, T. Saito, M. Dai, J. Am. Chem. Soc. 2021, 143, 16383–16387.
- 16H. Wynberg, Chem. Rev. 1960, 60, 169–184.
- 17R. Stenner, J. W. Steventon, A. Seddon, J. L. R. Anderson, Proc. Nat. Acad. Sci. 2020, 117, 1419–1428.
- 18B. D. Dherange, P. Q. Kelly, J. P. Liles, M. S. Sigman, M. D. Levin, J. Am. Chem. Soc. 2021, 143, 11337–11344.
- 19B. W. Joynson, G. R. Cumming, L. T. Ball, Angew. Chem. Int. Ed. 2023, 62, e202305081.
- 20H. Guo, S. Qiu, P. Xu, Angew. Chem. Int. Ed. 2023, e202317104. 10.1002/anie.202317104.
- 21
- 21aN. A. Meanwell, J. Med. Chem. 2018, 61, 5822–5880;
- 21bE. P. Gillis, K. J. Eastman, M. D. Hill, J. Med. Chem. 2015, 58, 8315–8359.
- 22
- 22aJ. Nie, H.-C. Guo, D. Cahard, J.-A. Ma, Chem. Rev. 2011, 111, 455–529;
- 22bX. Yang, T. Wu, R. J. Phipps, F. D. Toste, Chem. Rev. 2015, 115, 826–870.
- 23
- 23aY. Yang, S. Liu, S. Li, Z. Liu, P. Liao, P. Sivaguru, Y. Lu, J. Gao, X. Bi, Angew. Chem. Int. Ed. 2023, 62, e202214519;
- 23bY. Yang, Z. Liu, Q. Song, P. Sivaguru, G. Zanoni, K. Wang, Q. Bi, X. Bi, Chem Catal. 2022, 2, 563–577;
- 23cZ. Liu, Y. Yang, X. Jiang, Q. Song, G. Zanoni, S. Liu, X. Bi, Org. Chem. Front. 2022, 9, 2444–2452;
- 23dY. Wu, Q. Song, Y. Xa, Y. Ning, P. Sivaguru, X. Bi, Org. Chem. Front. 2023, 10, 4588–4592;
- 23eY. Ning, H. Chen, Y. Ning, J. Zhang, X. Bi, Angew. Chem. Int. Ed. 2024, 63, e202318072;
- 23fZ. Liu, P. Sivaguru, G. Zanoni, X. Bi, Acc. Chem. Res. 2022, 55, 1763–1781;
- 23gZ. Liu, H. Wang, P. Sivaguru, S. P. Nolan, Q. Song, W. Yu, J. Jiang, E. A. Anderson, X. Bi, Nat. Commun. 2022, 13, 1674;
- 23hZ. Liu, S. Cao, W. Yu, J. Wu, F. Yi, E. D. Anderson, X. Bi, Chem. 2020, 6, 2110–2124;
- 23iS. Liu, Y. Yang, Q. Song, Z. Liu, Y. Lu, Z. Wang, P. Sivaguru, X. Bi, Nat. Chem. 2024, 16, https://doi.org/10.1038/s41557-024-01468-2.
- 24Deposition numbers 2301825 (for 52′) and 2294652 (for 77) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 25Y. Lian, H. M. L. Davies, J. Am. Chem. Soc. 2010, 132, 440–441.
- 26H. M. L. Davies, J. J. Matasi, C. Thornley, Tetrahedron Lett. 1995, 36, 7205–7208.
- 27Y. Lian, H. M. Davies, Org. Lett. 2012, 14, 1934–1937.
- 28G. Yin, Y. Li, R.-H. Wang, J.-F. Li, X.-T. Xu, Y.-X. Luan, M. Ye, ACS Catal. 2021, 11, 4606–4612.
- 29Y.-D. Du, S. Wang, H.-W. Du, X.-Y. Chang, X.-Y. Chen, Y.-L. Li, W. Shu, Nat. Commun. 2023, 14, 5339.
- 30X. Li, H. Zhao, X. Chen, H. Jiang, M. Zhang, Org. Chem. Front. 2020, 7, 425–429.
- 31Y. Guo, N. Li, J. Li, X. Bi, Z. Gao, Y.-N. Duan, J. Xiao, Commun. Chem. 2023, 6, 26.
- 32H. Hou, H. Li, Y. Xu, D. Tang, Y. Han, C. Yan, X. Chen, S. Zhu, Tetrahedron. 2018, 74, 6577–6583.
- 33
- 33aZ. Liu, Y. Yang, Q. Song, L. Li, G. Zanoni, S. Liu, M. Xiang, E. D. Anderson, X. Bi, Nat. Commun. 2022, 13, 7649;
- 33bF.-Q. Shi, X. Li, Y. Xia, L. Zhang, Z.-X. Yu, J. Am. Chem. Soc. 2007, 129, 15503–15512.
- 34T. Lu, F. Chen, J. Comput. Chem. 2012, 33, 580–592.
- 35E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, J. Cohen, A. W. Yang, J. Am. Chem. Soc. 2010, 132, 6498–6506.
- 36T. Lu, Q. Chen, J. Comput. Chem. 2022, 43, 539–555.
- 37T. Lu, Z. Liu, Q. Chen, Mat. Sci. Eng. B. 2021, 273, 115425.