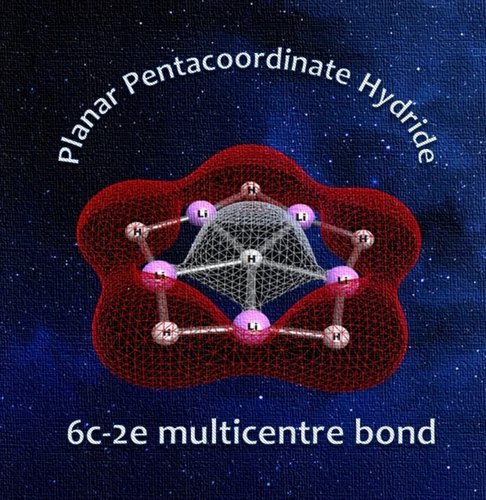
Pushing The Extreme of Multicentre Bonding: Planar Pentacoordinate Hydride
Graphical Abstract
Abstract
Planar hypercoordination has sparkled interest among the researchers from last few decades. Most of the elements in the Periodic Table have shown this remarkable structural feature. However, the smallest element, hydrogen, is missing in the list. No evidence is there in the literature. Herein, we introduce the first planar pentacoordinate hydrogen atom (ppH) in the global minimum geometry of Li5H6− cluster. Bonding analysis indicates that the central hydrogen atom is stabilized by multicentre bonding with five surrounding Li atoms. Natural charge analysis reveals that the central hydrogen is acting like a hydride which is strongly attracted by the positively charged surrounding lithium centres. The ppH structure is stabilized by strong electrostatic attraction as well as extensive multicentre bonding. Aromaticity has no role to play here. The cluster is dynamically stable and is expected to be detected in gas phase.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.