Eu(OTf)3-Catalyzed Formal Dipolar [4π+2σ] Cycloaddition of Bicyclo-[1.1.0]butanes with Nitrones: Access to Polysubstituted 2-Oxa-3-azabicyclo[3.1.1]heptanes
Corresponding Author
Dr. Jian Zhang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, 688 Yingbin Road, Jinhua, 321004 China
Search for more papers by this authorJia-Yi Su
State Key Laboratory of Bioreactor Engineering, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Search for more papers by this authorDr. Hanliang Zheng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, 688 Yingbin Road, Jinhua, 321004 China
Search for more papers by this authorProf. Dr. Hao Li
State Key Laboratory of Bioreactor Engineering, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Wei-Ping Deng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, 688 Yingbin Road, Jinhua, 321004 China
Search for more papers by this authorCorresponding Author
Dr. Jian Zhang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, 688 Yingbin Road, Jinhua, 321004 China
Search for more papers by this authorJia-Yi Su
State Key Laboratory of Bioreactor Engineering, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Search for more papers by this authorDr. Hanliang Zheng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, 688 Yingbin Road, Jinhua, 321004 China
Search for more papers by this authorProf. Dr. Hao Li
State Key Laboratory of Bioreactor Engineering, Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Wei-Ping Deng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, 688 Yingbin Road, Jinhua, 321004 China
Search for more papers by this authorGraphical Abstract
Abstract
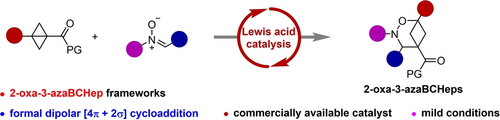
Herein, we have synthesized multifunctionalized 2-oxa-3-azabicyclo[3.1.1]heptanes, which are considered potential bioisosteres for meta-substituted arenes, through Eu(OTf)3-catalyzed formal dipolar [4π+2σ] cycloaddition of bicyclo[1.1.0]butanes with nitrones. This methodology represents the initial instance of fabricating bicyclo[3.1.1]heptanes adorned with multiple heteroatoms. The protocol exhibits both mild reaction conditions and a good tolerance for various functional groups. Computational density functional theory calculations support that the reaction mechanism likely involves a nucleophilic addition of nitrones to bicyclo[1.1.0]butanes, succeeded by an intramolecular cyclization. The synthetic utility of this novel protocol has been demonstrated in the concise synthesis of the analogue of Rupatadine.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202318476-sup-0001-checkcif.pdf86.4 KB | Supporting Information |
| anie202318476-sup-0001-misc_information.pdf10.5 MB | Supporting Information |
| anie202318476-sup-0001-mo_d8v23541_0m.cif862.4 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aF. Lovering, J. Bikker, C. Humblet, J. Med. Chem. 2009, 52, 6752–6756;
- 1bF. Lovering, MedChemComm 2013, 4, 515–519;
- 1cM. J. Caplin, D. J. Foley, Chem. Sci. 2021, 12, 4646–4660.
- 2
- 2aG. A. Patani, E. J. LaVoie, Chem. Rev. 1996, 96, 3147–3176;
- 2bN. A. Meanwell, J. Med. Chem. 2011, 54, 2529–2591;
- 2cP. K. Mykhailiuk, Org. Biomol. Chem. 2019, 17, 2839–2849;
- 2dM. R. Bauer, P. Di Fruscia, S. C. C. Lucas, I. N. Michaelides, J. E. Nelson, R. I. Storer, B. C. Whitehurst, RSC Med. Chem. 2021, 12, 448–471;
- 2eM. A. M. Subbaiah, N. A. Meanwell, J. Med. Chem. 2021, 64, 14046–14128.
- 3For selected recent reviews on BCPs synthesis:
- 3aJ. Kanazawa, M. Uchiyama, Synlett 2019, 30, 1–11;
- 3bX. Ma, L. N. Pham, Asian J. Org. Chem. 2020, 9, 8–22;
- 3cM. M. D. Pramanik, H. Qian, W.-J. Xiao, J.-R. Chen, Org. Chem. Front. 2020, 7, 2531–2537;
- 3dJ. M. Anderson, N. D. Measom, J. A. Murphy, D. L. Poole, Angew. Chem. Int. Ed. 2021, 60, 24754–24769.
- 4For selected examples on BCPs synthesis (not starting from BCBs):
- 4aR. Gianatassio, J. M. Lopchuk, J. Wang, C.-. M. Pan, L. R. Malins, L. Prieto, T. A. Brandt, M. R. Collins, G. M. Gallego, N. W. Sach, J. E. Spangler, H. Zhu, J. Zhu, P. S. Baran, Science 2016, 351, 241–246;
- 4bJ. Kanazawa, K. Maeda, M. Uchiyama, J. Am. Chem. Soc. 2017, 139, 17791–17794;
- 4cX. Zhang, R. T. Smith, C. Le, S. J. McCarver, B. T. Shireman, N. I. Carruthers, D. W. C. MacMillan, Nature 2020, 580, 220–226;
- 4dS. Yu, C. Jing, A. Noble, V. K. Aggarwal, Angew. Chem. Int. Ed. 2020, 59, 3917–3921;
- 4eZ. Wu, Y. Xu, J. Liu, X. Wu, C. Zhu, Sci. China Chem. 2020, 63, 1025–1029;
- 4fY. Yang, J. Tsien, J. M. E. Hughes, B. K. Peters, R. R. Merchant, T. Qin, Nat. Chem. 2021, 13, 950–955;
- 4gS. Shin, S. Lee, W. Choi, N. Kim, S. Hong, Angew. Chem. Int. Ed. 2021, 60, 7873–7879;
- 4hZ. Wu, Y. Xu, H. Zhang, X. Wu, C. Zhu, Chem. Commun. 2021, 57, 6066–6069;
- 4iW. Dong, E. Yen-Pon, L. Li, A. Bhattacharjee, A. Jolit, G. A. Molander, Nat. Chem. 2022, 14, 1068–1077;
- 4jW. Huang, S. Keess, G. A. Molander, J. Am. Chem. Soc. 2022, 144, 12961–12969;
- 4kS. Livesley, A. J. Sterling, C. M. Robertson, W. R. F. Goundry, J. A. Morris, F. Duarte, C. Aïssa, Angew. Chem. Int. Ed. 2022, 61, e202111291;
- 4lI. F. Yu, J. L. Manske, A. Diéguez-Vázquez, A. Misale, A. E. Pashenko, P. K. Mykhailiuk, S. V. Ryabukhin, D. M. Volochnyuk, J. F. Hartwig, Nat. Chem. 2023, 15, 685–693;
- 4mX. Zhao, J.-Y. Shou, F.-L. Qing, Sci. China Chem. 2023, 66, 2871–2877.
- 5For selected recent examples on BCHs synthesis (not starting from BCBs):
- 5aV. V. Levterov, Y. Panasyuk, V. O. Pivnytska, P. K. Mykhailiuk, Angew. Chem. Int. Ed. 2020, 59, 7161–7167;
- 5bA. Denisenko, P. Garbuz, S. V. Shishkina, N. M. Voloshchuk, P. K. Mykhailiuk, Angew. Chem. Int. Ed. 2020, 59, 20515–20521;
- 5cA. Denisenko, P. Garbuz, N. M. Voloshchuk, Y. Holota, G. Al-Maali, P. Borysko, P. K. Mykhailiuk, Nat. Chem. 2023, 15, 1155–1163;
- 5dA. Denisenko, P. Garbuz, Y. Makovetska, O. Shablykin, D. Lesyk, G. Al-Maali, R. Korzh, I. V. Sadkova, P. K. Mykhailiuk, Chem. Sci. 2023, 14, 14092–14099.
- 6For selected recent examples on BCHeps synthesis (not starting from BCBs):
- 6aJ. Zhao, J. L. Brosmer, Q. Tang, Z. Yang, K. N. Houk, P. L. Diaconescu, O. Kwon, J. Am. Chem. Soc. 2017, 139, 9807–9810;
- 6bA. S. Harmata, T. E. Spiller, M. J. Sowden, C. R. J. Stephenson, J. Am. Chem. Soc. 2021, 143, 21223–21228;
- 6cN. Frank, J. Nugent, B. R. Shire, H. D. Pickford, P. Rabe, A. J. Sterling, T. Zarganes-Tzitzikas, T. Grimes, A. L. Thompson, R. C. Smith, C. J. Schofield, P. E. Brennan, F. Duarte, E. A. Anderson, Nature 2022, 611, 721–726;
- 6dT. Iida, J. Kanazawa, T. Matsunaga, K. Miyamoto, K. Hirano, M. Uchiyama, J. Am. Chem. Soc. 2022, 144, 21848–21852;
- 6eR. Giovanelli, L. Lombardi, R. Pedrazzani, M. Monari, M. C. Reis, C. S. López, G. Bertuzzi, M. Bandini, Org. Lett. 2023, 25, 6969–6974.
- 7The example for the synthesis of hetro-BCHeps via nonBCB chemistry: D. Dibchak, M. Snisarenko, A. Mishuk, O. Shablykin, L. Bortnichuk, O. Klymenko-Ulianov, Y. Kheylik, I. V. Sadkova, H. S. Rzepa, P. K. Mykhailiuk, Angew. Chem. Int. Ed. 2023, e202304246.
- 8For reviews:
- 8aM. A. A. Walczak, T. Krainz, P. Wipf, Acc. Chem. Res. 2015, 48, 1149–1158;
- 8bA. Fawcett, Pure Appl. Chem. 2020, 92, 751–765;
- 8cC. B. Kelly, J. A. Milligan, L. J. Tilley, T. M. Sodano, Chem. Sci. 2022, 13, 11721–11737;
- 8dM. Golfmann, J. C. L. Walker, Commun. Chem. 2023, 6, 9; For recent examples on the ring-opening of BCBs:
- 8eZ. Zhang, V. Gevorgyan, J. Am. Chem. Soc. 2022, 144, 20875–20883;
- 8fH.-C. Shen, M. V. Popescu, Z.-S. Wang, L. de Lescure, A. Noble, R. S. Paton, V. K. Aggarwal, J. Am. Chem. Soc. 2023, 145, 16508–16516;
- 8gL. Tang, Q.-N. Huang, F. Wu, Y. Xiao, J.-L. Zhou, T.-T. Xu, W.-B. Wu, S. Qu, J.-J. Feng, Chem. Sci. 2023, 14, 9696–9703;
- 8hY. Xiao, T.-T. Xu, J.-L. Zhou, F. Wu, L. Tang, R.-Y. Liu, W.-B. Wu, J.-J. Feng, Chem. Sci. 2023, 14, 13060–13066.
- 9
- 9aD. E. Applequist, J. W. Wheeler, Tetrahedron Lett. 1977, 18, 3411–3412;
10.1016/S0040-4039(01)83253-6 Google Scholar
- 9bR. M. Bychek, V. Hutskalova, Y. P. Bas, O. A. Zaporozhets, S. Zozulya, V. V. Levterov, P. K. Mykhailiuk, J. Org. Chem. 2019, 84, 15106–15117;
- 9cX. Ma, D. L. Sloman, Y. Han, D. J. Bennett, Org. Lett. 2019, 21, 7199–7203;
- 9dX. Ma, W. Pinto, L. N. Pham, D. L. Sloman, Y. Han, Eur. J. Org. Chem. 2020, 2020, 4581–4605;
- 9eR. E. McNamee, M. M. Haugland, J. Nugent, R. Chan, K. E. Christensen, E. A. Anderson, Chem. Sci. 2021, 12, 7480–7485;
- 9fR. E. McNamee, A. L. Thompson, E. A. Anderson, J. Am. Chem. Soc. 2021, 143, 21246–21251;
- 9gR. Bychek, P. K. Mykhailiuk, Angew. Chem. Int. Ed. 2022, 61, e202205103.
- 10
- 10aA. Cairncross, E. P. Blanchard Jr, J. Am. Chem. Soc. 1966, 88, 496–504;
- 10bA. D. Meijere, H. Wenck, F. Seyed-Mahdavi, H. G. Viehe, V. Gallez, I. Erden, Tetrahedron 1986, 42, 1291–1297;
- 10cP. Wipf, M. A. A. Walczak, Angew. Chem. Int. Ed. 2006, 45, 4172–4175;
- 10dB. D. Schwartz, A. P. Smyth, P. E. Nashar, M. G. Gardiner, L. R. Malins, Org. Lett. 2022, 24, 1268–1273;
- 10eM. Wang, Y. Huang, C. Li, P. Lu, Org. Chem. Front. 2022, 9, 2149–2153.
- 11R. Kleinmans, T. Pinkert, S. Dutta, T. O. Paulisch, H. Keum, C. G. Daniliuc, F. Glorius, Nature 2022, 605, 477–482.
- 12R. Guo, Y.-C. Chang, L. Herter, C. Salome, S. E. Braley, T. C. Fessard, M. K. Brown, J. Am. Chem. Soc. 2022, 144, 7988–7994.
- 13
- 13aY. Liang, R. Kleinmans, C. G. Daniliuc, F. Glorius, J. Am. Chem. Soc. 2022, 144, 20207–20213;
- 13bM. Xu, Z. Wang, Z. Sun, Y. Ouyang, Z. Ding, T. Yu, L. Xu, P. Li, Angew. Chem. Int. Ed. 2022, 61, e202214507;
- 13cS. Agasti, F. Beltran, E. Pye, N. Kaltsoyannis, G. E. M. Crisenza, D. J. Procter, Nat. Chem. 2023, 15, 535–541;
- 13dR. Kleinmans, S. Dutta, K. Ozols, H. Shao, F. Schäfer, R. E. Thielemann, H. T. Chan, C. G. Daniliuc, K. N. Houk, F. Glorius, J. Am. Chem. Soc. 2023, 145, 12324–12332;
- 13eM. de Robichon, T. Kratz, F. Beyer, J. Zuber, C. Merten, T. Bach, J. Am. Chem. Soc. 2023, 145, 24466–24470;
- 13fY. Liu, S. Lin, Y. Li, J.-H. Xue, Q. Li, H. Wang, ACS Catal. 2023, 13, 5096–5103;
- 13gH. Yan, Y. Liu, X. Feng, L. Shi, Org. Lett. 2023, 25, 8116–8120.
- 14K. Dhake, K. J. Woelk, J. Becica, A. Un, S. E. Jenny, D. C. Leitch, Angew. Chem. Int. Ed. 2022, 61, e202204719.
- 15Y. Liang, F. Paulus, C. G. Daniliuc, F. Glorius, Angew. Chem. Int. Ed. 2023, 62, e202305043.
- 16N. Radhoff, C. G. Daniliuc, A. Studer, Angew. Chem. Int. Ed. 2023, 62, e202304771.
- 17D. Ni, S. Hu, X. Tan, Y. Yu, Z. Li, L. Deng, Angew. Chem. Int. Ed. 2023, e202308606.
- 18L. Tang, Y. Xiao, F. Wu, J.-L. Zhou, T.-T. Xu, J.-J. Feng, Angew. Chem. Int. Ed. 2023, e202310066.
- 19Y. Zheng, W. Huang, R. K. Dhungana, A. Granados, S. Keess, M. Makvandi, G. A. Molander, J. Am. Chem. Soc. 2022, 144, 23685–23690.
- 20T. Yu, J. Yang, Z. Wang, Z. Ding, M. Xu, J. Wen, L. Xu, P. Li, J. Am. Chem. Soc. 2023, 145, 4304–4310.
- 21T. V. T. Nguyen, A. Bossonnet, M. D. Wodrich, J. Waser, J. Am. Chem. Soc. 2023, 145, 25411–25421.
- 22
- 22aY.-Z. Liu, Z. Wang, Z. Huang, X. Zheng, W.-L. Yang, W.-P. Deng, Angew. Chem. Int. Ed. 2020, 59, 1238–1242;
- 22bJ. Zhang, Y.-S. Gao, B.-M. Gu, W.-L. Yang, B.-X. Tian, W.-P. Deng, ACS Catal. 2021, 11, 3810–3821;
- 22cW.-L. Yang, X.-Y. Shang, X. Luo, W.-P. Deng, Angew. Chem. Int. Ed. 2022, 61, e202203661;
- 22dW.-L. Yang, X.-Y. Shang, T. Ni, H. Yan, X. Luo, H. Zheng, Z. Li, W.-P. Deng, Angew. Chem. Int. Ed. 2022, 61, e202210207.
- 23Selected reviews on nitrones synthetic applications:
- 23aS.-I. Murahashi, Y. Imada, Chem. Rev. 2019, 119, 4684–4716;
- 23bN. Zou, X.-T. Qin, Z.-X. Wang, W.-M. Shi, D.-L. Mo, Chin. J. Org. Chem. 2021, 41, 4535–4553; selected examples:;
- 23cI. S. Young, M. A. Kerr, Angew. Chem. Int. Ed. 2003, 42, 3023–3026;
- 23dR. L. Sahani, R.-S. Liu, ACS Catal. 2019, 9, 5890–5896;
- 23eS. Zhou, Y. Li, X. Liu, W. Hu, Z. Ke, X. Xu, J. Am. Chem. Soc. 2021, 143, 14703–14711;
- 23fT.-Z. Li, S.-J. Liu, Y.-W. Sun, S. Deng, W. Tan, Y. Jiao, Y.-C. Zhang, F. Shi, Angew. Chem. Int. Ed. 2021, 60, 2355–2363;
- 23gJ. Qi, F. Wei, S. Huang, C.-H. Tung, Z. Xu, Angew. Chem. Int. Ed. 2021, 60, 4561–4565;
- 23hX. Zhong, M. Huang, H. Xiong, Y. Liang, W. Zhou, Q. Cai, Angew. Chem. Int. Ed. 2022, 61, e202208323;
- 23iJ.-Q. Zhang, P.-W. Qiu, C. Liang, D.-L. Mo, Org. Lett. 2022, 24, 7801–7805;
- 23jY. Xu, H.-X. Gao, C. Pan, Y. Shi, C. Zhang, G. Huang, C. Feng, Angew. Chem. Int. Ed. 2023, 62, e202310671.
- 24S. Clementson, A. Radaelli, K. Fjelbye, D. Tanner, M. Jessing, Org. Lett. 2019, 21, 4763–4766.
- 25Deposition numbers 2303432 (for 3 b) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 26R. Bosma, Z. Wang, A. J. Kooistra, N. Bushby, S. Kuhne, J. van den Bor, M. J. Waring, C. de Graaf, I. J. de Esch, H. F. Vischer, R. J. Sheppard, M. Wijtmans, R. Leurs, J. Med. Chem. 2019, 62, 6630–6644.