Revealing the Dominance of the Dissolution-Deposition Mechanism in Aqueous Zn−MnO2 Batteries
Yadong Li
College of Physics, Qingdao University, Qingdao, 266071 China
These authors contributed equally to this work.
Search for more papers by this authorYuhao Li
College of Physics, Qingdao University, Qingdao, 266071 China
These authors contributed equally to this work.
Search for more papers by this authorQingshan Liu
College of Physics, Qingdao University, Qingdao, 266071 China
Search for more papers by this authorYongshuai Liu
College of Physics, Qingdao University, Qingdao, 266071 China
Search for more papers by this authorTiansheng Wang
College of Physics, Qingdao University, Qingdao, 266071 China
Search for more papers by this authorMingjin Cui
Center of Energy Storage Materials & Technology, College of Engineering and Applied Sciences, Jiangsu Key Laboratory of Artificial Functional Materials, National Laboratory of Solid State Microstructures, and Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, 210093 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Yu Ding
Center of Energy Storage Materials & Technology, College of Engineering and Applied Sciences, Jiangsu Key Laboratory of Artificial Functional Materials, National Laboratory of Solid State Microstructures, and Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, 210093 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Hongsen Li
College of Physics, Qingdao University, Qingdao, 266071 China
Search for more papers by this authorCorresponding Author
Prof. Guihua Yu
Materials Science and Engineering Program and Walker Department of Mechanical Engineering, The University of Texas at Austin, Austin, TX 78712 USA
Search for more papers by this authorYadong Li
College of Physics, Qingdao University, Qingdao, 266071 China
These authors contributed equally to this work.
Search for more papers by this authorYuhao Li
College of Physics, Qingdao University, Qingdao, 266071 China
These authors contributed equally to this work.
Search for more papers by this authorQingshan Liu
College of Physics, Qingdao University, Qingdao, 266071 China
Search for more papers by this authorYongshuai Liu
College of Physics, Qingdao University, Qingdao, 266071 China
Search for more papers by this authorTiansheng Wang
College of Physics, Qingdao University, Qingdao, 266071 China
Search for more papers by this authorMingjin Cui
Center of Energy Storage Materials & Technology, College of Engineering and Applied Sciences, Jiangsu Key Laboratory of Artificial Functional Materials, National Laboratory of Solid State Microstructures, and Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, 210093 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Yu Ding
Center of Energy Storage Materials & Technology, College of Engineering and Applied Sciences, Jiangsu Key Laboratory of Artificial Functional Materials, National Laboratory of Solid State Microstructures, and Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, 210093 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Hongsen Li
College of Physics, Qingdao University, Qingdao, 266071 China
Search for more papers by this authorCorresponding Author
Prof. Guihua Yu
Materials Science and Engineering Program and Walker Department of Mechanical Engineering, The University of Texas at Austin, Austin, TX 78712 USA
Search for more papers by this authorGraphical Abstract
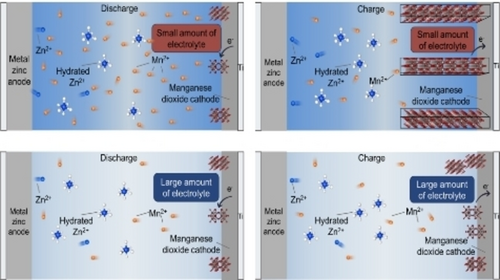
This work pinpointed the cause of the capacity degradation of Zn−MnO2 batteries and established the dominance of the MnO2/Mn2+ dissolution-deposition mechanism. Such a methodology circumvents the complicated characterization methods used in previous studies and provides a universal approach to accurately identify the reaction mechanism of other aqueous batteries.
Abstract
Zn−MnO2 batteries have attracted extensive attention for grid-scale energy storage applications, however, the energy storage chemistry of MnO2 in mild acidic aqueous electrolytes remains elusive and controversial. Using α-MnO2 as a case study, we developed a methodology by coupling conventional coin batteries with customized beaker batteries to pinpoint the operating mechanism of Zn−MnO2 batteries. This approach visually simulates the operating state of batteries in different scenarios and allows for a comprehensive study of the operating mechanism of aqueous Zn−MnO2 batteries under mild acidic conditions. It is validated that the electrochemical performance can be modulated by controlling the addition of Mn2+ to the electrolyte. The method is utilized to systematically eliminate the possibility of Zn2+ and/or H+ intercalation/de-intercalation reactions, thereby confirming the dominance of the MnO2/Mn2+ dissolution-deposition mechanism. By combining a series of phase and spectroscopic characterizations, the compositional, morphological and structural evolution of electrodes and electrolytes during battery cycling is probed, elucidating the intrinsic battery chemistry of MnO2 in mild acid electrolytes. Such a methodology developed can be extended to other energy storage systems, providing a universal approach to accurately identify the reaction mechanism of aqueous aluminum-ion batteries as well.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202318444-sup-0001-misc_information.pdf1.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Armand, J. M. Tarascon, Nature 2008, 451, 652;
- 1bD. Ma, H. Zhao, F. Cao, H. Zhao, J. Li, L. Wang, K. Liu, Chem. Sci. 2022, 13, 2385;
- 1cF. Li, Y. Li, L. Zhao, J. Liu, F. Zuo, F. Gu, H. Liu, R. Liu, Y. Li, J. Zhan, Q. Li, H. Li, Adv. Sci. 2022, 9, 2203895;
- 1dH. Yu, H. Zhou, J. Phys. Chem. Lett. 2013, 4, 1268;
- 1eZ. Zhao, H. Zhang, F. Li, L. Zhao, Q. Li, H. Li, Nano Lett. 2022, 22, 10102;
- 1fT. Koketsu, J. Ma, B. J. Morgan, M. Body, C. Legein, W. Dachraoui, M. Giannini, A. Demortiere, M. Salanne, F. Dardoize, H. Groult, O. J. Borkiewicz, K. Chapman, P. Strasser, D. Dambournet, Nat. Mater. 2017, 16, 1142.
- 2
- 2aS. Wang, S. Jiao, J. Wang, H.-S. Chen, D. Tian, H. Lei, D.-N. Fang, ACS Nano 2017, 11, 469;
- 2bY. Zhang, S. Liu, Y. Ji, J. Ma, H. Yu, Adv. Mater. 2018, 30, 1706310;
- 2cY.-S. Kim, K. D. Harris, B. Limoges, V. Balland, Chem. Sci. 2019, 10, 8752;
- 2dX. Wu, J. J. Hong, W. Shin, L. Ma, T. Liu, X. Bi, Y. Yuan, Y. Qi, T. W. Surta, W. Huang, J. Neuefeind, T. Wu, P. A. Greaney, J. Lu, X. Ji, Nat. Energy 2019, 4, 123;
- 2eK. Xu, C. Wang, Nat. Energy 2016, 1, 16161;
- 2fF. Wang, O. Borodin, T. Gao, X. Fan, W. Sun, F. Han, A. Faraone, J. A. Dura, K. Xu, C. Wang, Nat. Mater. 2018, 17, 543.
- 3
- 3aX. Wang, Y. Wang, A. Naveed, G. Li, H. Zhang, Y. Zhou, A. Dou, M. Su, Y. Liu, R. Guo, C. C. Li, Adv. Funct. Mater. 2023, 33, 2306205;
- 3bS. Kim, G. Piao, D. S. Han, H. K. Shon, H. Park, Energy Environ. Sci. 2018, 11, 344;
- 3cH. Wang, L. Zhao, H. Zhang, Y. Liu, L. Yang, F. Li, W. Liu, X. Dong, X. Li, Z. Li, X. Qi, L. Wu, Y. Xu, Y. Wang, K. Wang, H. Yang, Q. Li, S. Yan, X. Zhang, F. Li, H. Li, Energy Environ. Sci. 2022, 15, 311;
- 3dM.-C. Lin, M. Gong, B. Lu, Y. Wu, D.-Y. Wang, M. Guan, M. Angell, C. Chen, J. Yang, B.-J. Hwang, H. Dai, Nature 2015, 520, 325;
- 3eH. Xia, X. Zhu, J. Liu, Q. Liu, S. Lan, Q. Zhang, X. Liu, J. K. Seo, T. Chen, L. Gu, Y. S. Meng, Nat. Commun. 2018, 9, 5100;
- 3fH. Pan, Y. Shao, P. Yan, Y. Cheng, K. S. Han, Z. Nie, C. Wang, J. Yang, X. Li, P. Bhattacharya, K. T. Mueller, J. Liu, Nat. Energy 2016, 1, 160399;
- 3gY. Liu, Y. Li, F. Zuo, J. Liu, Y. Xu, L. Yang, H. Zhang, H. Wang, X. Zhang, C. Liu, Q. Li, H. Li, Small 2022, 18, 2203236.
- 4X. Wu, X. Ji, Nat. Chem. 2019, 11, 680.
- 5
- 5aL. Ding, L. Wang, J. Gao, T. Yan, H. Li, J. Mao, F. Song, S. Fedotov, L.-Y. Chang, N. Li, Y. Su, T. Liu, L. Zhang, Adv. Funct. Mater. 2023, 33, 2301648;
- 5bS. Shen, D. Ma, K. Ouyang, Y. Chen, M. Yang, Y. Wang, S. Sun, H. Mi, L. Sun, C. He, P. Zhang, Adv. Funct. Mater. 2023, 33, 2304255;
- 5cM. Song, H. Tan, D. Chao, H. J. Fan, Adv. Funct. Mater. 2018, 28, 1802564;
- 5dZ. Liu, X. Luo, L. Qin, G. Fang, S. Liang, Adv. Powder Mater. 2022, 1, 1802564;
- 5eZ. Liu, Y. Huang, Y. Huang, Q. Yang, X. Li, Z. Huang, C. Zhi, Chem. Soc. Rev. 2020, 49, 180;
- 5fD. Chao, W. Zhou, F. Xie, C. Ye, H. Li, M. Jaroniec, S.-Z. Qiao, Sci. Adv. 2020, 6, eaba4098.
- 6
- 6aH. Wang, H. Du, R. Zhao, Z. Zhu, L. Qie, J. Fu, Y. Huang, Adv. Funct. Mater. 2023, 33, 2213803;
- 6bJ. Hao, X. Li, X. Zeng, D. Li, J. Mao, Z. Guo, Energy Environ. Sci. 2020, 13, 3917.
- 7S. Wu, Z. Hu, P. He, L. Ren, J. Huang, J. Luo, eScience 2023, 3, 100120.
- 8
- 8aG. G. Yadav, D. Turney, J. Huang, X. Wei, S. Banerjee, ACS Energy Lett. 2019, 4, 2144;
- 8bY. Jin, L. Zou, L. Liu, M. H. Engelhard, R. L. Patel, Z. Nie, K. S. Han, Y. Shao, C. Wang, J. Zhu, H. Pan, J. Liu, Adv. Mater. 2019, 31, 1900567;
- 8cH. Ren, J. Zhao, L. Yang, Q. Liang, S. Madhavi, Q. Yan, Nano Res. 2019, 12, 1347;
- 8dC. Wu, S. Gu, Q. Zhang, Y. Bai, M. Li, Y. Yuan, H. Wang, X. Liu, Y. Yuan, N. Zhu, F. Wu, H. Li, L. Gu, J. Lu, Nat. Commun. 2019, 10, 73;
- 8eC. Zhong, B. Liu, J. Ding, X. Liu, Y. Zhong, Y. Li, C. Sun, X. Han, Y. Deng, N. Zhao, W. Hu, Nat. Energy 2020, 5, 440.
- 9
- 9aW. Sun, F. Wang, S. Hou, C. Yang, X. Fan, Z. Ma, T. Gao, F. Han, R. Hu, M. Zhu, C. Wang, J. Am. Chem. Soc. 2017, 139, 9775;
- 9bQ. Zhao, X. Chen, Z. Wang, L. Yang, R. Qin, J. Yang, Y. Song, S. Ding, M. Weng, W. Huang, J. Liu, W. Zhao, G. Qian, K. Yang, Y. Cui, H. Chen, F. Pan, Small 2019, 15, 1904545.
- 10
- 10aC. Wei, J. Song, Y. Wang, X. Tang, X. Liu, Adv. Funct. Mater. 2023, 33, 2304223;
- 10bH. Chen, S. Cai, Y. Wu, W. Wang, M. Xu, S. J. Bao, Mater. Today Energy 2021, 20, 100646;
- 10cJ. Huang, Z. Guo, X. Dong, D. Bin, Y. Wang, Y. Xia, Sci. Bull. 2019, 64, 1780.
- 11
- 11aX. Chen, P. Ruan, X. Wu, S. Liang, J. Zhou, Acta Phys.-Chim. Sin. 2022, 38, 2111003;
- 11bD. Chao, C. Ye, F. Xie, W. Zhou, Q. Zhang, Q. Gu, K. Davey, L. Gu, S.-Z. Qiao, Adv. Mater. 2020, 32, 2001894;
- 11cH. Chen, C. Dai, F. Xiao, Q. Yang, S. Cai, M. Xu, H. J. Fan, S.-J. Bao, Adv. Mater. 2022, 34, 2109092;
- 11dD. Wu, L. M. Housel, S. T. King, Z. R. Mansley, N. Sadique, Y. Zhu, L. Ma, S. N. Ehrlich, H. Zhong, E. S. Takeuchi, A. C. Marschilok, D. C. Bock, L. Wang, K. J. Takeuchi, J. Am. Chem. Soc. 2022, 144, 23405.
- 12
- 12aD. Wu, L. M. Housel, S. J. Kim, N. Sadique, C. D. Quilty, L. Wu, R. Tappero, S. L. Nicholas, S. Ehrlich, Y. Zhu, A. C. Marschilok, E. S. Takeuchi, D. C. Bock, K. J. Takeuchi, Energy Environ. Sci. 2020, 13, 4322;
- 12bX. Guo, J. Zhou, C. Bai, X. Li, G. Fang, S. Liang, Mater. Today Energy 2020, 16, 100396.
- 13C. F. Bischoff, O. S. Fitz, J. Burns, M. Bauer, H. Gentischer, K. P. Birke, H.-M. Henning, D. Biro, J. Electrochem. Soc. 2020, 167, 020545.
- 14Y. Yuan, R. Sharpe, K. He, C. Li, M. T. Saray, T. Liu, W. Yao, M. Cheng, H. Jin, S. Wang, K. Amine, R. Shahbazian-Yassar, M. S. Islam, J. Lu, Nat. Sustainability 2022, 5, 890.
- 15Z. Jia, J. Hao, L. Liu, Y. Wang, T. Qi, Ionics 2018, 24, 3483.
- 16T. N. T. Tran, S. Jin, M. Cuisinier, B. D. Adams, D. G. Ivey, Sci. Rep. 2021, 11, 20777.
- 17
- 17aV. Soundharrajan, B. Sambandam, S. Kim, S. Islam, J. Jo, S. Kim, V. Mathew, Y.-k. Sun, J. Kim, Energy Storage Mater. 2020, 28, 407;
- 17bN. Zhang, F. Cheng, J. Liu, L. Wang, X. Long, X. Liu, F. Li, J. Chen, Nat. Commun. 2017, 8, 405.
- 18H. Yang, W. Zhou, D. Chen, J. Liu, Z. Yuan, M. Lu, L. Shen, V. Shulga, W. Han, D. Chao, Energy Environ. Sci. 2022, 15, 1106.
- 19S. Bi, Y. Wu, A. Cao, J. Tian, S. Zhang, Z. Niu, Mater. Today Energy 2020, 18,100548.
- 20
- 20aS. He, J. Wang, X. Zhang, J. Chen, Z. Wang, T. Yang, Z. Liu, Y. Liang, B. Wang, S. Liu, L. Zhang, J. Huang, J. Huang, L. A. O'Dell, H. Yu, Adv. Funct. Mater. 2019, 29, 1905228;
- 20bC. Julien, M. Massot, R. Baddour-Hadjean, S. Franger, S. Bach, J. P. Pereira-Ramos, Solid State Ionics 2003, 159, 345.
- 21
- 21aH. Yang, T. Zhang, D. Chen, Y. Tan, W. Zhou, L. Li, W. Li, G. Li, W. Han, H. J. Fan, D. Chao, Adv. Mater. 2023, 35, 2300053;
- 21bB. Sambandam, V. Mathew, S. Kim, S. Lee, S. Kim, J. Y. Hwang, H. J. Fan, J. Kim, Chem 2022, 8, 924.