Boundary-Rich Carbon-Based Electrocatalysts with Manganese(II)-Coordinated Active Environment for Selective Synthesis of Hydrogen Peroxide
Ling-Yu Dong
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
These authors contributed equally to this work.
Search for more papers by this authorJing-Song Wang
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
These authors contributed equally to this work.
Search for more papers by this authorTian-Yi Li
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
Search for more papers by this authorTao Wu
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
Search for more papers by this authorXu Hu
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
Search for more papers by this authorYu-Tai Wu
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
Search for more papers by this authorMin-Yi Zhu
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Guang-Ping Hao
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
Search for more papers by this authorCorresponding Author
Prof. An-Hui Lu
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
Search for more papers by this authorLing-Yu Dong
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
These authors contributed equally to this work.
Search for more papers by this authorJing-Song Wang
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
These authors contributed equally to this work.
Search for more papers by this authorTian-Yi Li
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
Search for more papers by this authorTao Wu
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
Search for more papers by this authorXu Hu
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
Search for more papers by this authorYu-Tai Wu
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
Search for more papers by this authorMin-Yi Zhu
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Guang-Ping Hao
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
Search for more papers by this authorCorresponding Author
Prof. An-Hui Lu
State Key Laboratory of Fine Chemicals, Frontier Science Center for Smart Materials, Liaoning Key Laboratory for Catalytic Conversion of Carbon Resources, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024 Liaoning, P. R. China
Search for more papers by this authorGraphical Abstract
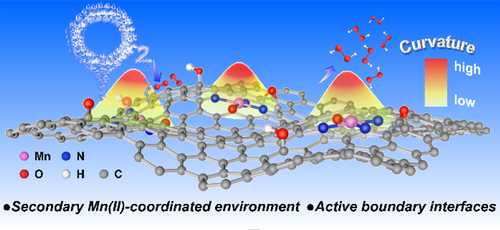
Carbon-supported manganese-centered boundary-rich electrocatalysts show high activity for selective H2O2 synthesis. The secondary epoxide and hydroxyl groups surrounding Mn−N3−O sites and the O2 enrichment in the curved boundaries together contribute to the activity enhancement for the targeted electrosynthesis.
Abstract
Coordinated manganese (Mn) electrocatalysts owing to their electronic structure flexibility, non-toxic and earth abundant features are promising for electrocatalytic reactions. However, achieving selective hydrogen peroxide (H2O2) production through two electron oxygen reduction (2e-ORR) is a challenge on Mn-centered catalysts. Targeting this goal, we report on the creation of a secondary Mn(II)-coordinated active environment with reactant enrichment effect on boundary-rich porous carbon-based electrocatalysts, which facilitates the selective and rapid synthesis of H2O2 through 2e-ORR. The catalysts exhibit nearly 100 % Faradaic efficiency and H2O2 productivity up to 15.1 mol gcat−1 h−1 at 0.1 V versus reversible hydrogen electrode, representing the record high activity for Mn-based electrocatalyst in H2O2 electrosynthesis. Mechanistic studies reveal that the epoxide and hydroxyl groups surrounding Mn(II) centers improve spin state by modifying electronic properties and charge transfer, thus tailoring the adsorption strength of *OOH intermediate. Multiscale simulations reveal that the high-curvature boundaries facilitate oxygen (O2) adsorption and result in local O2 enrichment due to the enhanced interaction between carbon surface and O2. These merits together ensure the efficient formation of H2O2 with high local concentration, which can directly boost the tandem reaction of hydrolysis of benzonitrile to benzamide with nearly 100 % conversion rate and exclusive benzamide selectivity.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202317660-sup-0001-misc_information.pdf5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. Xia, Y. Xia, P. Zhu, L. Fan, H. Wang, Science 2019, 366, 226–231.
- 2
- 2aM. Volokh, M. Shalom, Nat. Catal. 2021, 4, 350–351;
- 2bS. Yang, A. Verdaguer-Casadevall, L. Arnarson, L. Silvioli, V. Čolić, R. Frydendal, J. Rossmeisl, I. Chorkendorff, I. E. L. Stephens, ACS Catal. 2018, 8, 4064–4081;
- 2cJ. Sun, H. Sekhar Jena, C. Krishnaraj, K. Singh Rawat, S. Abednatanzi, J. Chakraborty, A. Laemont, W. Liu, H. Chen, Y. Liu, K. Leus, H. Vrielinck, V. Van Speybroeck, P. Van Der Voort, Angew. Chem. Int. Ed. 2023, 62, e202216719.
- 3X. Zhang, X. Su, Y. Zheng, S. Hu, L. Shi, F. Gao, P. Yang, Z. Niu, Z. Wu, S. Qin, R. Wu, Y. Duan, C. Gu, X. Zheng, J. Zhu, M. Gao, Angew. Chem. Int. Ed. 2021, 60, 26922–26931.
- 4S. Siahrostami, S. J. Villegas, A. H. Bagherzadeh Mostaghimi, S. Back, A. B. Farimani, H. Wang, K. A. Persson, J. Montoya, ACS Catal. 2020, 10, 7495–7511.
- 5J. Feng, H. Gao, L. Zheng, Z. Chen, S. Zeng, C. Jiang, H. Dong, L. Liu, S. Zhang, X. Zhang, Nat. Commun. 2020, 11, 4341.
- 6X. Bai, Y. Wang, J. Han, X. Niu, J. Guan, Appl. Catal. B 2023, 337, 122966.
- 7Y. Zhu, W. Wang, J. Cheng, Y. Qu, Y. Dai, M. Liu, J. Yu, C. Wang, H. Wang, S. Wang, C. Zhao, Y. Wu, Y. Liu, Angew. Chem. Int. Ed. 2021, 60, 9480–9488.
- 8B. Yang, H. Yao, J. Yang, C. Chen, Y. Guo, H. Fu, J. Shi, J. Am. Chem. Soc. 2022, 144, 314–330.
- 9S. Qiao, D. Lei, Q. Wang, X. Shi, Q. Zhang, C. Huang, A. Liu, G. He, F. Zhang, Chem. Eng. J. 2022, 442, 136258.
- 10
- 10aE. Antico, M. Leutzsch, N. Wessel, T. Weyhermüller, C. Werlé, W. Leitner, Chem. Sci. 2023, 14, 54–60;
- 10bH. Wu, X. Li, C. Tung, L. Wu, Chem. Commun. 2020, 56, 15496–15512;
- 10cE. Edwardes Moore, S. J. Cobb, A. M. Coito, A. R. Oliveira, I. A. C. Pereira, E. Reisner, Proc. Nat. Acad. Sci. 2022, 119, e2114097119.
- 11M. Gennari, D. Brazzolotto, J. Pécaut, M. V. Cherrier, C. J. Pollock, S. DeBeer, M. Retegan, D. A. Pantazis, F. Neese, M. Rouzières, R. Clérac, C. Duboc, J. Am. Chem. Soc. 2015, 137, 8644–8653.
- 12S. L. Hooe, A. L. Rheingold, C. W. Machan, J. Am. Chem. Soc. 2018, 140, 3232–3241.
- 13S. L. Hooe, C. W. Machan, J. Am. Chem. Soc. 2019, 141, 4379–4387.
- 14J. Li, M. Chen, D. A. Cullen, S. Hwang, M. Wang, B. Li, K. Liu, S. Karakalos, M. Lucero, H. Zhang, C. Lei, H. Xu, G. E. Sterbinsky, Z. Feng, D. Su, K. L. More, G. Wang, Z. Wang, G. Wu, Nat. Catal. 2018, 1, 935–945.
- 15H. Shang, W. Sun, R. Sui, J. Pei, L. Zheng, J. Dong, Z. Jiang, D. Zhou, Z. Zhuang, W. Chen, J. Zhang, D. Wang, Y. Li, Nano Lett. 2020, 20, 5443–5450.
- 16
- 16aK. Sun, J. Dong, H. Sun, X. Wang, J. Fang, Z. Zhuang, S. Tian, X. Sun, Nat. Catal. 2023, 6, 1164–1173;
- 16bX. Peng, Y. Mi, X. Liu, J. Sun, Y. Qiu, S. Zhang, X. Ke, X. Wang, J. Luo, J. Mater. Chem. A 2022, 10, 6134–6145;
- 16cG. Wei, Y. Li, X. Liu, J. Huang, M. Liu, D. Luan, S. Gao, X. D. Lou, Angew. Chem. Int. Ed. 2023, 62, e202313914;
- 16dH. Y. F. Sim, J. R. T. Chen, C. S. L. Koh, H. K. Lee, X. Han, G. C. Phan-Quang, J. Y. Pang, C. L. Lay, S. Pedireddy, I. Y. Phang, E. K. L. Yeow, X. Y. Ling, Angew. Chem. Int. Ed. 2020, 59, 16997–17003;
- 16eM. Yu, G. Moon, R. G. Castillo, S. DeBeer, C. Weidenthaler, H. Tüysüz, Angew. Chem. Int. Ed. 2020, 59, 16544–16552.
- 17C. Tang, L. Chen, H. Li, L. Li, Y. Jiao, Y. Zheng, H. Xu, K. Davey, S. Qiao, J. Am. Chem. Soc. 2021, 143, 7819–7827.
- 18
- 18aB. Lee, H. Shin, A. S. Rasouli, H. Choubisa, P. Ou, R. Dorakhan, I. Grigioni, G. Lee, E. Shirzadi, R. K. Miao, J. Wicks, S. Park, H. S. Lee, J. Zhang, Y. Chen, Z. Chen, D. Sinton, T. Hyeon, Y. Sung, E. H. Sargent, Nat. Catal. 2023, 6, 234–243;
- 18bA. Kumar, Y. Zhang, W. Liu, X. Sun, Coord. Chem. Rev. 2020, 402, 213047.
- 19P. Cao, X. Quan, X. Nie, K. Zhao, Y. Liu, S. Chen, H. Yu, J. G. Chen, Nat. Commun. 2023, 14, 172.
- 20
- 20aQ. Zhang, X. Tan, N. M. Bedford, Z. Han, L. Thomsen, S. Smith, R. Amal, X. Lu, Nat. Commun. 2020, 11, 4181;
- 20bB. Zhang, T. Zheng, Y. Wang, Y. Du, S. Chu, Z. Xia, R. Amal, S. Dou, L. Dai, Comm. Chem. 2022, 5, 43;
- 20cM. Jin, S. Liu, G. Meng, S. Zhang, Q. Liu, J. Luo, X. Liu, Adv. Mater. Interfaces 2023, 10, 2201144.
- 21
- 21aH. Xu, J. Chen, Z. Zhang, C. Hung, J. Yang, W. Li, Adv. Mater. 2023, 35, 2207522;
- 21bK. Dong, J. Liang, Y. Wang, Z. Xu, Q. Liu, Y. Luo, T. Li, L. Li, X. Shi, A. M. Asiri, Q. Li, D. Ma, X. Sun, Angew. Chem. Int. Ed. 2021, 60, 10583–10587;
- 21cY. Zhang, H. Jang, X. Ge, W. Zhang, Z. Li, L. Hou, L. Zhai, X. Wei, Z. Wang, M. G. Kim, S. Liu, Q. Qin, X. Liu, J. Cho, Adv. Energy Mater. 2022, 12, 2202695;
- 21dJ. Wordsworth, T. M. Benedetti, S. V. Somerville, W. Schuhmann, R. D. Tilley, J. J. Gooding, Angew. Chem. Int. Ed. 2022, 61, e202200755.
- 22
- 22aM. Luo, Z. Zhao, Y. Zhang, Y. Sun, Y. Xing, F. Lv, Y. Yang, X. Zhang, S. Hwang, Y. Qin, J. Ma, F. Lin, D. Su, G. Lu, S. Guo, Nature 2019, 574, 81–85;
- 22bG. Chen, S. K. Singh, K. Takeyasu, J. P. Hill, J. Nakamura, K. Ariga, Sci. Technol. Adv. Mater. 2022, 23, 413–423;
- 22cJ. Su, C. B. Musgrave, Y. Song, L. Huang, Y. Liu, G. Li, Y. Xin, P. Xiong, M. M. Li, H. Wu, M. Zhu, H. M. Chen, J. Zhang, H. Shen, B. Z. Tang, M. Robert, W. A. Goddard, R. Ye, Nat. Catal. 2023, 6, 818–828;
- 22dM. Jin, W. Liu, J. Sun, X. Wang, S. Zhang, J. Luo, X. Liu, Nano Res. 2022, 15, 5842–5847.
- 23
- 23aC. Liu, L. Gui, J. Zheng, Y. Xu, B. Song, L. Yi, Y. Jia, A. Taledaohan, Y. Wang, X. Gao, Z. Qiao, H. Wang, Z. Tang, J. Am. Chem. Soc. 2023, 145, 19086–19097;
- 23bH. Guo, L. Li, Y. Chen, W. Zhang, C. Shang, X. Cao, M. Li, Q. Zhang, H. Tan, Y. Nie, L. Gu, S. Guo, Adv. Mater. 2023, 35, 2302285;
- 23cF. Gao, S. Hu, X. Zhang, Y. Zheng, H. Wang, Z. Niu, P. Yang, R. Bao, T. Ma, Z. Dang, Y. Guan, X. Zheng, X. Zheng, J. Zhu, M. Gao, S. Yu, Angew. Chem. Int. Ed. 2020, 59, 8706–8712;
- 23dZ. Yu, N. Ji, J. Xiong, X. Li, R. Zhang, L. Zhang, X. Lu, Angew. Chem. Int. Ed. 2021, 60, 20786–20794.
- 24Z. Guo, Y. Xie, J. Xiao, Z. Zhao, Y. Wang, Z. Xu, Y. Zhang, L. Yin, H. Cao, J. Gong, J. Am. Chem. Soc. 2019, 141, 12005–12010.
- 25B. Zhang, J. Zhang, J. Shi, D. Tan, L. Liu, F. Zhang, C. Lu, Z. Su, X. Tan, X. Cheng, B. Han, L. Zheng, J. Zhang, Nat. Commun. 2019, 10, 2980.
- 26
- 26aJ. Guan, Z. Duan, F. Zhang, S. D. Kelly, R. Si, M. Dupuis, Q. Huang, J. Q. Chen, C. Tang, C. Li, Nat. Catal. 2018, 1, 870–877;
- 26bA. Zitolo, V. Goellner, V. Armel, M. Sougrati, T. Mineva, L. Stievano, E. Fonda, F. Jaouen, Nat. Mater. 2015, 14, 937–942;
- 26cL. Bai, Z. Duan, X. Wen, R. Si, J. Guan, Appl. Catal. B 2019, 257, 117930.
- 27V. Gridin, J. Du, S. Haller, P. Theis, K. Hofmann, G. K. H. Wiberg, U. I. Kramm, M. Arenz, Electrochim. Acta 2023, 444, 142012.
- 28S. Chen, T. Luo, X. Li, K. Chen, J. Fu, K. Liu, C. Cai, Q. Wang, H. Li, Y. Chen, C. Ma, L. Zhu, Y. Lu, T. Chan, M. Zhu, E. Cortés, M. Liu, J. Am. Chem. Soc. 2022, 144, 14505–14516.
- 29H. Jin, P. Li, P. Cui, J. Shi, W. Zhou, X. Yu, W. Song, C. Cao, Nat. Commun. 2022, 13, 723.
- 30P. A. Andreev, Mod. Phys. Lett. B 2017, 31, 1750152.
- 31Z. Chen, H. Niu, J. Ding, H. Liu, P. Chen, Y. Lu, Y. Lu, W. Zuo, L. Han, Y. Guo, S. Hung, Y. Zhai, Angew. Chem. Int. Ed. 2021, 60, 25404–25410.
- 32Q. Zhao, Y. Wang, W. Lai, F. Xiao, Y. Lyu, C. Liao, M. Shao, Energy Environ. Sci. 2021, 14, 5444–5456.
- 33Y. Wang, R. Shi, L. Shang, G. I. N. Waterhouse, J. Zhao, Q. Zhang, L. Gu, T. Zhang, Angew. Chem. Int. Ed. 2020, 59, 13057–13062.
- 34K. Jiang, S. Back, A. J. Akey, C. Xia, Y. Hu, W. Liang, D. Schaak, E. Stavitski, J. K. Nørskov, S. Siahrostami, H. Wang, Nat. Commun. 2019, 10, 3997.
- 35P. Cao, X. Quan, K. Zhao, X. Zhao, S. Chen, H. Yu, ACS Catal. 2021, 11, 13797–13808.