Photoredox-Catalyzed Generation of Tertiary Anions from Primary Amines via a Radical Polar Crossover
Austin D. Marchese
Department of Chemistry, Columbia University, New York, NY 10027 USA
Search for more papers by this authorJulia R. Dorsheimer
Department of Chemistry, Columbia University, New York, NY 10027 USA
Search for more papers by this authorCorresponding Author
Prof. Tomislav Rovis
Department of Chemistry, Columbia University, New York, NY 10027 USA
Search for more papers by this authorAustin D. Marchese
Department of Chemistry, Columbia University, New York, NY 10027 USA
Search for more papers by this authorJulia R. Dorsheimer
Department of Chemistry, Columbia University, New York, NY 10027 USA
Search for more papers by this authorCorresponding Author
Prof. Tomislav Rovis
Department of Chemistry, Columbia University, New York, NY 10027 USA
Search for more papers by this authorGraphical Abstract
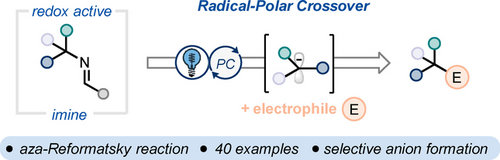
A deaminative radical-polar crossover strategy generating carbanions is reported. This so called “aza-Reformatsky” reaction leverages the use of redox-active imines and an iridium photocatalyst to generate a wide array of tertiary benzylic, heteroarylbenzylic and α-ester anions, which can be trapped with different electrophiles.
Abstract
A method for the generation of tertiary carbanions via a deaminative radical-polar crossover is reported using redox active imines from α-tertiary primary amines. A variety of benzylic amines and amino esters can be used in this approach, with the latter engaging in a novel “aza-Reformatsky” reaction. Electronic trends correlate the stability of the resulting carbanion with reaction efficiency. The anions can be trapped with different electrophiles including aldehydes, ketones, imines, Michael acceptors, and H2O/D2O. Selective anion formation can be achieved in the presence of another equivalent or more acidic C−H bond in both an inter- and intramolecular fashion. Mechanistic studies suggest the intermediacy of a discrete carbanion intermediate.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202317563-sup-0001-misc_information.pdf10 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. Z. Ali, B. G. Budaitis, D. F. Fontaine, A. L. Pace, J. A. Garwin, M. C. White, Science 2022, 376, 276–283.
- 2A. Hager, N. Vrielink, D. Hager, J. Lefranc, D. Trauner, Nat. Prod. Rep. 2016, 33, 491–522.
- 3A recent analysis of the >400,000 building blocks on the eMolecule database showed that primary amines are the most prevalent functional group, appearing in ≈13 % of the molecules available. Reference: Y. Zabolotna, D. M. Volochnyuk, S. V. Ryabukhin, D. Horvath, K. S. Gavrilenko, G. Marcou, Y. S. Moroz, O. Oksiuta, A. Varnek, J. Chem. Inf. Model. 2022, 62, 2171–218.
- 4K. Ouyang, W. Hao, W. X. Zhang, Z. Xi, Chem. Rev. 2015, 115, 12045–12090.
- 5Y. Jin, H. Fu, J. Org. Chem. 2017, 6, 68–385.
- 6Y. Wang, J. Xu, Y. Pan, Y. Wang, Org. Biomol. Chem. 2023, 21, 1121–1133.
- 7C. Zhu, H. Yue, L. Chu, M. Rueping, Chem. Sci. 2020, 11, 4051–4064.
- 8S. Davey. Nat. Chem. 2010, https://doi.org/10.1038/nchem.867.
10.1038/nchem.867 Google Scholar
- 9Y. Kato, D. H. Yen, Y. Fukudome, T. Hata, H. Urabe, Org. Lett. 2010, 12, 4137–4139.
- 10
- 10aB. D. Dherange, M. Yuan, C. B. Kelly, C. A. Reiher, C. Grosanu, K. J. Berger, O. Gutierrez, M. D. Levin, J. Am. Chem. Soc. 2023, 145, 17–24;
- 10bD. Chattapadhyay, A. Aydogan, K. Doktor, A. Maity, J. W. Wu, Q. Michaudel, ACS Catal. 2023, 13, 7263–7268;
- 10cK. A. Steiniger, M. C. Lamb, T. H. Lambert, J. Am. Chem. Soc. 2023, 145, 11524–11529;
- 10dS. P. Lathrop, M. Movassaghi, Chem. Sci. 2014, 5, 333–340.
- 11J. T. M. Correia, V. A. Fernandes, B. T. Matsuo, J. A. C. Delgado, W. C. De Souza, M. W. Paixão, Chem. Commun. 2020, 56, 503–514.
- 12Y. Gao, S. Jiang, N. D. Mao, H. Xiang, J. L. Duan, X. Y. Ye, L. W. Wang, Y. Ye, T. Xie, Top. Curr. Chem. 2022, 380, 25.
- 13M. A. Ashley, T. Rovis, J. Am. Chem. Soc. 2020, 142, 18310–18316.
- 14J. R. Dorsheimer, M. A. Ashley, T. Rovis, J. Am. Chem. Soc. 2021, 143, 19294–19299.
- 15J. Liao, C. H. Basch, M. E. Hoerrner, M. R. Talley, B. P. Boscoe, J. W. Tucker, M. R. Garnsey, M. P. Watson, Org. Lett. 2019, 21, 2941–2946.
- 16S. Tcyrulnikov, Q. Cai, J. Cameron Twitty, J. Xu, A. Atifi, O. P. Bercher, G. P. A. Yap, J. Rosenthal, M. P. Watson, M. C. Kozlowski, ACS Catal. 2021, 11, 8456–8466.
- 17L. Huang, R. Kancherla, M. Rueping, ACS Catal. 2022, 12, 11563–11572.
- 18S. Sharma, J. Singh, A. Sharma, Adv. Synth. Catal. 2021, 363, 3146–3169.
- 19R. J. Wiles, G. A. Molander, Isr. J. Chem. 2020, 60, 281–293.
- 20L. Pitzer, J. L. Schwarz, F. Glorius, Chem. Sci. 2019, 10, 8285–8291.
- 21K. Donabauer, B. König, Acc. Chem. Res. 2021, 54, 242–252.
- 22G. Liu, Y. Gao, W. Su, J. Org. Chem. 2023, 88, 6322–6332.
- 23K. Donabauer, M. Maity, A. L. Berger, G. S. Huff, S. Crespi, B. König, Chem. Sci. 2019, 10, 5162–5166.
- 24K. Donabauer, K. Murugesan, U. Rozman, S. Crespi, B. König, Chem. Eur. J. 2020, 26, 12945–12950.
- 25For examples of photocatalytic generation of 3° anions where CO2 was used as an electrophile see:
- 25aL. L. Liao, G. M. Cao, J. H. Ye, G. Q. Sun, W. J. Zhou, Y. Y. Gui, S. S. Yan, G. Shen, D.-G. Yu, J. Am. Chem. Soc. 2018, 140, 17338–17342;
- 25bC. K. Ran, Y. N. Niu, L. Song, M. K. Wei, Y. F. Cao, S. P. Luo, Y. M. Yu, L. L. Liao, D.-G. Yu, ACS Catal. 2022, 12, 18–24;
- 25cD. Kong, M. Munch, Q. Qiqige, C. J. Cooze, B. H. Rotstein, R. J. Lundgren, J. Am. Chem. Soc. 2021, 143, 2200–2206.
- 26For electrochemical examples of 3° anion formation, see:
- 26aW. Guan, Y. Chang, S. Lin, J. Am. Chem. Soc. 2023, 145, 16966–16972;
- 26bW. Zhang, L. Lu, W. Zhang, Y. Wang, S. D. Ware, J. Mondragon, J. Rein, N. Strotman, D. Lehnherr, K. A. See, S. Lin, Nature 2022, 604, 292–297.
- 27C. Hansch, A. Leo, R. W. A. Taft, Chem. Rev. 1991, 91, 165–195.
- 28T. P. Bender, J. F. Graham, J. M. Duff, Chem. Mater. 2001, 13, 4105–4111.
- 29S. Nagamine, E. Horisaka, Y. Fukuyama, K. Maetani, R. Matsuzawa, S. Iwakawa, S. Asada, Biol. Pharm. Bull. 1997, 20, 188–192.
- 30S. J. Hays, M. C. Tobes, D. L. Gildersleeve, D. M. Wieland, W. H. Beierwaltes, J. Med. Chem. 1984, 27, 15–19.
- 31F. J. R. Klauck, M. J. James, F. Glorius, Angew. Chem. Int. Ed. 2017, 56, 12336–12339.
- 32M. E. Hoerrner, K. M. Baker, C. H. Basch, E. M. Bampo, M. P. Watson, Org. Lett. 2019, 21, 7356–7360.
- 33Z. F. Zhu, M. M. Zhang, F. Liu, Org. Biomol. Chem. 2019, 17, 1531–1534.
- 34
- 34aS.-Z. Sun, C. Romano, R. Martin, J. Am. Chem. Soc. 2019, 141, 16197–16201;
- 34bS.-Z. Sun, Y.-M. Cai, D.-L. Zhang, J.-B. Wang, H.-Q. Yao, X.-Y. Rui, R. Martin, M. Shang, J. Am. Chem. Soc. 2022, 144, 1130–1137.
- 35G. B. Watson, T. H. Lanthorn, Neuropharmacology 1990, 29, 727–730.
- 36M. Concheiro, R. Chesser, G. Cooper, Front. Pharmacol. 2018, 9, 1210.
- 37
- 37aY. Ohshima, K. Kaira, A. Yamaguchi, N. Oriuchi, H. Tominaga, S. Nagamori, Y. Kanai, T. Yokobori, T. Miyazaki, T. Asao, Y. Tsushima, H. Kuwano, N. S. Ishioka, Cancer Sci. 2016, 107, 1499–1505;
- 37bN. Singh, M. Scalise, M. Galluccio, M. Wieder, T. Seidel, T. Langer, C. Indiveri, G. F. Ecker, Int. J. Mol. Sci. 2019, 20, https://doi.org/10.3390/ijms20010027.
10.3390/ijms20010027 Google Scholar
- 38Oxidation of the product results in one compound, confirming the epimer of the isolated product is of the benzylic alcohol stereocenter. 2D NMR analysis suggests that the obtained isomer is the one stemming from the approach of the aldehyde to the more accessible exo-face of the norbornane, in line with additions of analogous norbornane-derived enolate nucleophiles; see:
- 38aJ. Ibarzo, R. M. Ortuño, Tetrahedron 1994, 50, 9825–9830;
- 38bA. P. Krapcho, E. A. Dundulis, J. Org. Chem. 1980, 45, 3236–3245. See Supporting Information for complete characterization.
- 39Previous DFT analysis suggested that oxidation of the trimethoxyarene ring acidifies the other α-amino proton to a greater extent than the imidoyl proton. Deprotonation occurs competitively at this position, generating an unproductive aza-allyl radical. See: M. A. Ashley, Photoredox-Catalyzed Site-Selective Functionalization of Primary Amine Derivatives, Doctoral dissertation, Columbia University, 2020.
- 40R. Ocampo, W. R. Dolbier, Tetrahedron 2004, 60, 9325–9374.
- 41M. Sailer, K. I. Dubicki, J. L. Sorensen, Synthesis 2015, 47, 79–82.
- 42R. Moumne, S. Lavielle, P. Karoyan, J. Org. Chem. 2006, 71, 3332–3334.
- 43E. Speckmeier, T. G. Fischer, K. A. Zeitler, J. Am. Chem. Soc. 2018, 140, 15353–15365.
- 44
- 44aN. P. Ramirez, J. C. Gonzalez-Gomez, Eur. J. Org. Chem. 2017, 2154–2163;
- 44bN. Bortolamei, A. A. Isse, A. Gennaro, Electrochim. Acta 2010, 55, 8312–8318.
- 45S. Kundu, L. Roy, M. S. Maji, Org. Lett. 2022, 24, 9001–9006.
- 46L. J. Rono, H. G. Yayla, D. Y. Wang, M. F. Armstrong, R. R. Knowles, J. Am. Chem. Soc. 2013, 135, 17735–17738.