Diastereo- and Enantioselective Synthesis of Tetracyclic Cycloheptanols through (4+3) Annulation via C−C/C−H Activation Cascade
Xin Yan
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, Sichuan, China, 610066
These authors contributed equally to this work.
Search for more papers by this authorMin Liu
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, Sichuan, China, 610066
These authors contributed equally to this work.
Search for more papers by this authorDeng Pan
Department of Chemistry, School of Science and Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Tianjin University, Tianjin, China
These authors contributed equally to this work.
Search for more papers by this authorQi Wang
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, Sichuan, China, 610066
Search for more papers by this authorQi Tang
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, Sichuan, China, 610066
Search for more papers by this authorYa-Mei Dai
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, Sichuan, China, 610066
Search for more papers by this authorProf. Ping Hu
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, Sichuan, China, 610066
Search for more papers by this authorBi-Qin Wang
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, Sichuan, China, 610066
Search for more papers by this authorCorresponding Author
Prof. Dr. Genping Huang
Department of Chemistry, School of Science and Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Tianjin University, Tianjin, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Feijie Song
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, Sichuan, China, 610066
Search for more papers by this authorXin Yan
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, Sichuan, China, 610066
These authors contributed equally to this work.
Search for more papers by this authorMin Liu
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, Sichuan, China, 610066
These authors contributed equally to this work.
Search for more papers by this authorDeng Pan
Department of Chemistry, School of Science and Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Tianjin University, Tianjin, China
These authors contributed equally to this work.
Search for more papers by this authorQi Wang
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, Sichuan, China, 610066
Search for more papers by this authorQi Tang
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, Sichuan, China, 610066
Search for more papers by this authorYa-Mei Dai
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, Sichuan, China, 610066
Search for more papers by this authorProf. Ping Hu
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, Sichuan, China, 610066
Search for more papers by this authorBi-Qin Wang
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, Sichuan, China, 610066
Search for more papers by this authorCorresponding Author
Prof. Dr. Genping Huang
Department of Chemistry, School of Science and Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Tianjin University, Tianjin, China
Search for more papers by this authorCorresponding Author
Prof. Dr. Feijie Song
College of Chemistry and Materials Science, Sichuan Normal University, Chengdu, Sichuan, China, 610066
Search for more papers by this authorGraphical Abstract
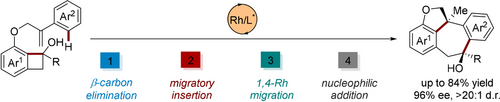
A Rhodium-catalyzed intramolecular (4+3) annulation of α-arylalkene–benzocyclobutenols has been developed, which proceeds through sequential β-carbon elimination of alcohols, migratory insertion with alkenes, 1,4-Rh shift, and nucleophilic addition to the in situ formed ketones. The protocol offers a diastereo- and enantioselective approach to dihydrofuran-annulated dibenzocycloheptanols with two discontinuous chiral carbon centers built.
Abstract
Transition metal-catalyzed annulations of four-membered rings via C−C activation are powerful tools to construct complex fused and bridged ring systems. Despite significant progress in (4+1), (4+2) and (4+4) annulations, the (4+3) annulation remains unexplored. Herein, we develop an asymmetric Rh-catalyzed intramolecular (4+3) annulation of α-arylalkene-tethered benzocyclobutenols for the synthesis of dihydrofuran-annulated dibenzocycloheptanols with two discontinuous chiral carbon centers via a C−C and C−H activation cascade. The reaction features excellent diastereo- and enantioselectivities and 100 % atom economy, and is applicable to late-stage modification of complex molecules.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202317433-sup-0001-misc_information.pdf26.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected recent reviews, see:
- 1aD.-S. Kim, W.-J. Park, C.-H. Jun, Chem. Rev. 2017, 117, 8977–9015;
- 1bF. Song, T. Gou, B.-Q. Wang, Z.-J. Shi, Chem. Soc. Rev. 2018, 47, 7078–7115;
- 1cT. R. McDonald, L. R. Mills, M. S. West, S. A. L. Rousseaux, Chem. Rev. 2021, 121, 3–79;
- 1dO. O. Sokolova, J. F. Bower, Chem. Rev. 2021, 121, 80–109;
- 1eR. Vicente, Chem. Rev. 2021, 121, 162–226;
- 1fM. D. R. Lutz, B. Morandi, Chem. Rev. 2021, 121, 300–326.
- 2For selected reviews, see:
- 2aL. Souillart, N. Cramer, Chem. Rev. 2015, 115, 9410–9464;
- 2bY. Wang, Z.-X. Yu, Acc. Chem. Res. 2015, 48, 2288–2296;
- 2cP.-H. Chen, B. A. Billett, T. Tsukamoto, G. Dong, ACS Catal. 2017, 7, 1340–1360;
- 2dJ. Wang, S. A. Blaszczyk, X. Li, W. Tang, Chem. Rev. 2021, 121, 110–139;
- 2eM. Font, M. Gulías, J. L. Mascareñas, Angew. Chem. Int. Ed. 2022, 61, e202112848.
- 3For selected recent reviews, see:
- 3aP. Chen, G. Dong, Chem. Eur. J. 2016, 22, 18290–18315;
- 3bM. Murakami, N. Ishida, Chem. Rev. 2021, 121, 264–299;
- 3cY. Xue, G. Dong, Acc. Chem. Res. 2022, 55, 2341–2354.
- 4
- 4aY. Xia, Z. Liu, Z. Liu, R. Ge, F. Ye, M. Hossain, Y. Zhang, J. Wang, J. Am. Chem. Soc. 2014, 136, 3013–3015;
- 4bA. Yada, S. Fujita, M. Murakami, J. Am. Chem. Soc. 2014, 136, 7217–7220;
- 4cX. Zhou, G. Dong, J. Am. Chem. Soc. 2015, 137, 13715–13721;
- 4dS. Ochi, Z. Zhang, Y. Xia, G. Dong, Angew. Chem. Int. Ed. 2022, 61, e202202703.
- 5
- 5aM. Murakami, S. Ashida, T. Matsuda, J. Am. Chem. Soc. 2006, 128, 2166–2167;
- 5bD. Crépin, J. Dawick, C. Aïssa, Angew. Chem. Int. Ed. 2010, 49, 620–623;
- 5cA. Thakur, M. E. Facer, J. Louie, Angew. Chem. Int. Ed. 2013, 52, 12161–12165;
- 5dF. Juliá-Hernández, A. Ziadi, A. Nishimura, R. Martin, Angew. Chem. Int. Ed. 2015, 54, 9537–9541;
- 5eS. Okumura, F. Sun, N. Ishida, M. Murakami, J. Am. Chem. Soc. 2017, 139, 12414–12417;
- 5fJ. Zhang, X. Wang, T. Xu, Nat. Commun. 2021, 12, 3022–3029.
- 6
- 6aA. Chtchemelinine, D. Rosa, A. Orellana, J. Org. Chem. 2011, 76, 9157–9162;
- 6bW. Mao, C. Zhu, J. Org. Chem. 2017, 82, 9133–9143;
- 6cL. Liu, F. Cheng, C. Meng, A.-A. Zhang, M. Zhang, K. Xu, N. Ishida, M. Murakami, ACS Catal. 2021, 11, 8942–8947.
- 7
- 7aN. Ishida, S. Sawano, Y. Masuda, M. Murakami, J. Am. Chem. Soc. 2012, 134, 17502–17504;
- 7bJ. Yu, H. Yan, C. Zhu, Angew. Chem. Int. Ed. 2016, 55, 1143–1146;
- 7cY. Zhao, P. Wang, Y. Gao, C. Zhu, W. Liu, Y. Wang, Org. Chem. Front. 2019, 6, 791–795.
- 8N. Ishida, N. Ishikawa, S. Sawano, Y. Masuda, M. Murakami, Chem. Commun. 2015, 51, 1882–1885.
- 9C. Zhao, L.-C. Liu, J. Wang, C. Jiang, Q.-W. Zhang, W. He, Org. Lett. 2016, 18, 328–331.
- 10Y. He, C. Yuan, Z. Jiang, L. Shuai, Q. Xiao, Org. Lett. 2019, 21, 185–189.
- 11
- 11aT.-M. Tang, M. Liu, H. Wu, T. Gou, X. Hu, B.-Q. Wang, P. Hu, F. Song, G. Huang, Org. Chem. Front. 2021, 8, 3867–3875;
- 11bY.-M. Dai, M. Liu, Q.-Q. Zeng, X. Li, B.-Q. Wang, P. Hu, K.-Q. Zhao, F. Song, Z.-J. Shi, Org. Lett. 2021, 23, 7597–7602;
- 11cQ.-Q. Zeng, Y.-Q. Wang, L. Cheng, B.-Q. Wang, P. Hu, F. Song, Org. Lett. 2022, 24, 3058–3063;
- 11dL. Cheng, Q. Tang, Y.-M. Dai, B.-Q. Wang, P. Hu, P. Cao, F. Song, ACS Catal. 2023, 13, 4362–4368.
- 12For reviews of metal-catalyzed transformations involving the activations of both C−C and C−H bonds, see:
- 12aZ. Nairoukh, M. Cormier, I. Marek, Nat. Chem. Rev. 2017, 1, 35–51;
- 12bH. Azizollahi, J.-A. García-López, Molecules 2020, 25, 5900–5966. For representative examples, see:
- 12cT. Matsuda, M. Shigeno, M. Murakami, J. Am. Chem. Soc. 2007, 129, 12086–12087;
- 12dT. Seiser, O. A. Roth, N. Cramer, Angew. Chem. Int. Ed. 2009, 48, 6320–6323;
- 12eM. Shigeno, T. Yamamoto, M. Murakami, Chem. Eur. J. 2009, 15, 12929–12931.
- 13
- 13aJ. Shen, Q. Zhou, P. Li, Z. Wang, S. Liu, C. He, C. Zhang, P. Xiao, Molecules 2017, 22, 2050–2075;
- 13bT. Ito, T. Tanaka, M. Iinuma, K.-I. Nakaya, Y. Takahashi, R. Sawa, J. Murata, D. Darnaedi, J. Nat. Prod. 2004, 67, 932–937;
- 13cT. M. Ngoc, T. M. Hung, P. T. Thuong, M. Na, H. Kim, D. T. Ha, B.-S. Min, P. T. H. Minh, K. Bae, Biol. Pharm. Bull. 2008, 31, 1809–1812;
- 13dT. Ito, R. Hoshino, M. Iinuma, Helv. Chim. Acta 2015, 98, 32–46;
- 13eK. Ninomiya, S. Chaipech, Y. Kunikata, R. Yagi, Y. Pongpiriyadacha, O. Muraoka, T. Morikawa, Int. J. Mol. Sci. 2017, 18, 451–464.
- 14Deposition numbers 2240329 (for 3 a), 2240342 (for 3 w), 2240343 (for (−)-3 a), 2240327 (for (±)-7) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 15For recent reviews of 1,4-metal shift, see:
- 15aA. Rahim, J. Feng, Z. Gu, Chin. J. Chem. 2019, 37, 929–945;
- 15bM.-Y. Li, D. Wei, C.-G. Feng, G.-Q. Lin, Chem. Asian J. 2022, 17, e202200456 . For selected examples, see:
- 15cQ. Tian, R. C. Larock, Org. Lett. 2000, 2, 3329–3332;
- 15dT. Hayashi, K. Inoue, N. Taniguchi, M. Ogasawara, J. Am. Chem. Soc. 2001, 123, 9918–9919;
- 15eM. Tobisu, I. Hyodo, M. Onoeb, N. Chatani, Chem. Commun. 2008, 6013–6015;
- 15fA. Clemenceau, P. Thesmar, M. Gicquel, A. L. Flohic, O. Baudoin, J. Am. Chem. Soc. 2020, 142, 15355–15361.
- 16
- 16aA. Kina, H. Iwamura, T. Hayashi, J. Am. Chem. Soc. 2006, 128, 3904–3905;
- 16bJ. Zhang, W. Xu, M.-H. Xu, Angew. Chem. Int. Ed. 2023, 62, e202216799.
- 17
- 17aJ. Panteleev, F. Menard, M. Lautens, Adv. Synth. Catal. 2008, 350, 2893–2902;
- 17bK. Sasaki, T. Nishimura, R. Shintani, E. A. B. Kantchev, T. Hayashi, Chem. Sci. 2012, 3, 1278–1283;
- 17cJ. Ming, T. Hayashi, Org. Lett. 2016, 18, 6452–6455;
- 17dN. Liu, J. Yao, L. Yin, T. Lu, Z. Tian, X. Dou, ACS Catal. 2019, 9, 6857–6863.
- 18
- 18aC. Bour, J. Suffert, Org. Lett. 2005, 7, 653–656;
- 18bA. J. Mota, A. Dedieu, C. Bour, J. Suffert, J. Am. Chem. Soc. 2005, 127, 7171–7182;
- 18cY. Ikeda, K. Takano, S. Kodama, Y. Ishii, Chem. Commun. 2013, 49, 11104–11106.
- 19L. Ding, N. Ishida, M. Murakami, K. Morokuma, J. Am. Chem. Soc. 2014, 136, 169–178.