Vitamin B12-Photocatalyzed Cyclopropanation of Electron-Deficient Alkenes Using Dichloromethane as the Methylene Source**
John Hayford G. Teye-Kau
Department of Chemistry, Oklahoma State University, 107 Physical Sciences, Stillwater, OK 74078 USA
Search for more papers by this authorMayokun J. Ayodele
Weaver Labs LLC, 1110 S. Innovation Way Dr., #130, Stillwater, OK 74074 USA
Search for more papers by this authorCorresponding Author
Prof. Spencer P. Pitre
Department of Chemistry, Oklahoma State University, 107 Physical Sciences, Stillwater, OK 74078 USA
Search for more papers by this authorJohn Hayford G. Teye-Kau
Department of Chemistry, Oklahoma State University, 107 Physical Sciences, Stillwater, OK 74078 USA
Search for more papers by this authorMayokun J. Ayodele
Weaver Labs LLC, 1110 S. Innovation Way Dr., #130, Stillwater, OK 74074 USA
Search for more papers by this authorCorresponding Author
Prof. Spencer P. Pitre
Department of Chemistry, Oklahoma State University, 107 Physical Sciences, Stillwater, OK 74078 USA
Search for more papers by this authorA previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.26434/chemrxiv-2023-m3shw).
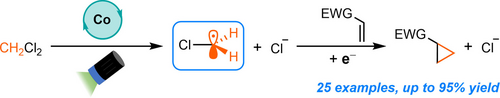
Graphical Abstract
We report a vitamin B12-photocatalyzed strategy for the cyclopropanation of electron-deficient alkenes using dichloromethane (CH2Cl2) as the methylene source. The reaction has excellent functional group tolerance, is highly chemoselective, and the scope can be extended to other 1,1-dichloroalkanes for the preparation of D2-cyclopropyl and methyl-substituted cyclopropyl adducts, all of which are important isosteres in medical chemistry.
Abstract
The cyclopropyl group is of great importance in medicinal chemistry, as it can be leveraged to influence a range of pharmaceutical properties in drug molecules. This report describes a Vitamin B12-photocatalyzed approach for the cyclopropanation of electron-deficient alkenes using dichloromethane (CH2Cl2) as the methylene source. The reaction proceeds in good to excellent yields under mild conditions, has excellent functional group compatibility, and is highly chemoselective. The scope could also be extended to the preparation of D2-cyclopropyl and methyl-substituted cyclopropyl adducts starting from CD2Cl2 and 1,1-dichloroethane, respectively.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202316064-sup-0001-misc_information.pdf4.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. Burger, in Prog. Drug Res. (Eds.: S. Sharma, S. Cohen, A. M. Karrow, M. W. Riley, R. P. Alquist, J. W. McFarland, H. Uehleke, O. Wintersteiner, A. Burger, E. R. Garret, E. Jucker), Birkhäuser Basel, Basel, 1971, pp. 227–270.
- 2T. T. Talele, J. Med. Chem. 2016, 59, 8712–8756.
- 3M. R. Bauer, P. Di Fruscia, S. C. C. Lucas, I. N. Michaelides, J. E. Nelson, R. I. Storer, B. C. Whitehurst, RSC Med. Chem. 2021, 12, 448–471.
- 4M. L. Landry, J. J. Crawford, ACS Med. Chem. Lett. 2020, 11, 72–76.
- 5“Drugbank Online. Chemical Structure Search,” can be found under https://go.drugbank.com/structures/search/small_molecule_drugs/structure#results.
- 6R. G. Gentles, M. Ding, J. A. Bender, C. P. Bergstrom, K. Grant-Young, P. Hewawasam, T. Hudyma, S. Martin, A. Nickel, A. Regueiro-Ren, Y. Tu, Z. Yang, K.-S. Yeung, X. Zheng, S. Chao, J.-H. Sun, B. R. Beno, D. M. Camac, C.-H. Chang, M. Gao, P. E. Morin, S. Sheriff, J. Tredup, J. Wan, M. R. Witmer, D. Xie, U. Hanumegowda, J. Knipe, K. Mosure, K. S. Santone, D. D. Parker, X. Zhuo, J. Lemm, M. Liu, L. Pelosi, K. Rigat, S. Voss, Y. Wang, Y.-K. Wang, R. J. Colonno, M. Gao, S. B. Roberts, Q. Gao, A. Ng, N. A. Meanwell, J. F. Kadow, J. Med. Chem. 2014, 57, 1855–1879.
- 7H.-U. Reissig, R. Zimmer, Chem. Rev. 2003, 103, 1151–1196.
- 8M. Rubin, M. Rubina, V. Gevorgyan, Chem. Rev. 2007, 107, 3117–3179.
- 9C. Ebner, E. M. Carreira, Chem. Rev. 2017, 117, 11651–11679.
- 10W. Wu, Z. Lin, H. Jiang, Org. Biomol. Chem. 2018, 16, 7315–7329.
- 11H. E. Simmons, R. D. Smith, J. Am. Chem. Soc. 1958, 80, 5323–5324.
- 12H. E. Simmons, R. D. Smith, J. Am. Chem. Soc. 1959, 81, 4256–4264.
- 13H. E. Simmons, T. L. Cairns, S. A. Vladuchick, C. M. Hoiness, in Organic Reactions, 2011, pp. 1–131.
- 14A. B. Charette, A. Beauchemin, in Organic Reactions, 2004, pp. 1–415.
- 15Y. G. Gololobov, A. N. Nesmeyanov, V. P. Lysenko, I. E. Boldeskul, Tetrahedron 1987, 43, 2609–2651.
- 16A.-H. Li, L.-X. Dai, V. K. Aggarwal, Chem. Rev. 1997, 97, 2341–2372.
- 17G. L. Beutner, D. T. George, Org. Process Res. Dev. 2023, 27, 10–41.
- 18Y. Chen, J. V. Ruppel, X. P. Zhang, J. Am. Chem. Soc. 2007, 129, 12074–12075.
- 19W.-C. C. Lee, X. P. Zhang, Trends Chem. 2022, 4, 850–851.
- 20J. Wang, J. Xie, W.-C. Cindy Lee, D.-S. Wang, X. P. Zhang, Chem Catal. 2022, 2, 330–344.
- 21X. Wang, J. Ke, Y. Zhu, A. Deb, Y. Xu, X. P. Zhang, J. Am. Chem. Soc. 2021, 143, 11121–11129.
- 22Y. Wang, X. Wen, X. Cui, L. Wojtas, X. P. Zhang, J. Am. Chem. Soc. 2017, 139, 1049–1052.
- 23X. Xu, Y. Wang, X. Cui, L. Wojtas, X. P. Zhang, Chem. Sci. 2017, 8, 4347–4351.
- 24Z.-L. Chen, Y. Xie, J. Xuan, Eur. J. Org. Chem. 2022, e202201066.
- 25A. M. del Hoyo, A. G. Herraiz, M. G. Suero, Angew. Chem. Int. Ed. 2017, 56, 1610–1613.
- 26A. M. del Hoyo, M. García Suero, Eur. J. Org. Chem. 2017, 2122–2125.
- 27J. P. Phelan, S. B. Lang, J. S. Compton, C. B. Kelly, R. Dykstra, O. Gutierrez, G. A. Molander, J. Am. Chem. Soc. 2018, 140, 8037–8047.
- 28C.-C. Tsai, I.-L. Hsieh, T.-T. Cheng, P.-K. Tsai, K.-W. Lin, T.-H. Yan, Org. Lett. 2006, 8, 2261–2263.
- 29Y.-Y. Zhou, C. Uyeda, Angew. Chem. Int. Ed. 2016, 55, 3171–3175.
- 30J. Werth, C. Uyeda, Angew. Chem. Int. Ed. 2018, 57, 13902–13906.
- 31J. Werth, K. Berger, C. Uyeda, Adv. Synth. Catal. 2020, 362, 348–352.
- 32K. E. Berger, R. J. Martinez, J. Zhou, C. Uyeda, J. Am. Chem. Soc. 2023, 145, 9441–9447.
- 33M. Liu, N. Le, C. Uyeda, Angew. Chem. Int. Ed. 2023, 62, e202308913.
- 34R. Scheffold, M. Dike, S. Dike, T. Herold, L. Walder, J. Am. Chem. Soc. 1980, 102, 3642–3644.
- 35B. Giese, P. Erdmann, T. Göbel, R. Springer, Tetrahedron Lett. 1992, 33, 4545–4548.
- 36M. E. Weiss, L. M. Kreis, A. Lauber, E. M. Carreira, Angew. Chem. Int. Ed. 2011, 50, 11125–11128.
- 37K. ó Proinsias, A. Jackowska, K. Radzewicz, M. Giedyk, D. Gryko, Org. Lett. 2018, 20, 296–299.
- 38S. W. Smoleń Aleksandra, O. Drapała, D. Gryko, Synthesis 2020, 53, 1645–1653.
- 39K. R. Dworakowski, S. Pisarek, S. Hassan, D. Gryko, Org. Lett. 2021, 23, 9068–9072.
- 40D. J. Charboneau, E. L. Barth, N. Hazari, M. R. Uehling, S. L. Zultanski, ACS Catal. 2020, 10, 12642–12656.
- 41C. K. Njue, B. Nuthakki, A. Vaze, J. M. Bobbitt, J. F. Rusling, Electrochem. Commun. 2001, 3, 733–736.
- 42G. N. Schrauzer, E. Deutsch, R. J. Windgassen, J. Am. Chem. Soc. 1968, 90, 2441–2442.
- 43G. N. Schrauzer, E. Deutsch, J. Am. Chem. Soc. 1969, 91, 3341–3350.
- 44H. Shimakoshi, Y. Maeyama, T. Kaieda, T. Matsuo, E. Matsui, Y. Naruta, Y. Hisaeda, Bull. Chem. Soc. Jpn. 2005, 78, 859–863.
- 45G. N. Schrauzer, J. W. Sibert, R. J. Windgassen, J. Am. Chem. Soc. 1968, 90, 6681–6688.
- 46T. H. Wright, B. J. Bower, J. M. Chalker, G. J. L. Bernardes, R. Wiewiora, W.-L. Ng, R. Raj, S. Faulkner, M. R. J. Vallée, A. Phanumartwiwath, O. D. Coleman, M.-L. Thézénas, M. Khan, S. R. G. Galan, L. Lercher, M. W. Schombs, S. Gerstberger, M. E. Palm-Espling, A. J. Baldwin, B. M. Kessler, T. D. W. Claridge, S. Mohammed, B. G. Davis, Science 2016, 354, aag1465.
- 47J. W. Bogart, A. A. Bowers, Org. Biomol. Chem. 2019, 17, 3653–3669.
- 48W.-C. C. Lee, D.-S. Wang, C. Zhang, J. Xie, B. Li, X. P. Zhang, Chem 2021, 7, 1588–1601.
- 49J. Hu, J. Wang, T. H. Nguyen, N. Zheng, Beilstein J. Org. Chem. 2013, 9, 1977–2001.
- 50J. W. Beatty, C. R. J. Stephenson, Acc. Chem. Res. 2015, 48, 1474–1484.
- 51F. Parsaee, M. C. Senarathna, P. B. Kannangara, S. N. Alexander, P. D. E. Arche, E. R. Welin, Nat. Chem. Rev. 2021, 5, 486–499.
- 52N. Rao, R. Kini, P. Kad, Pharm. Chem. J. 2022, 55, 1372–1377.
- 53C. Schmidt, Nat. Biotechnol. 2017, 35, 493–494.
- 54S. H. DeWitt, B. E. Maryanoff, Biochemistry 2018, 57, 472–473.
- 55H. Schönherr, T. Cernak, Angew. Chem. Int. Ed. 2013, 52, 12256–12267.
- 56Y. Chen, X. P. Zhang, J. Org. Chem. 2004, 69, 2431–2435.
- 57S. P. Pitre, T. K. Allred, L. E. Overman, Org. Lett. 2021, 23, 1103–1106.
- 58Y. Murakami, Y. Hisaeda, A. Kajihara, T. Ohno, Bull. Chem. Soc. Jpn. 1984, 57, 405–411.
- 59H. Shimakoshi, E. Sakumori, K. Kaneko, Y. Hisaeda, Chem. Lett. 2009, 38, 468–469.
- 60G. N. Schrauzer, L.-P. Lee, J. W. Sibert, J. Am. Chem. Soc. 1970, 92, 2997–3005.
- 61P. J. H. Williams, G. A. Boustead, D. E. Heard, P. W. Seakins, A. R. Rickard, V. Chechik, J. Am. Chem. Soc. 2022, 144, 15969–15976.
- 62T. Wdowik, D. Gryko, ACS Catal. 2022, 12, 6517–6531.