Stereospecific Radical Polymerization of a Side-Chain Transformable Bulky Acrylamide Monomer and Subsequent Post-Polymerization Modification for Syntheses of Isotactic Polyacrylate and Polyacrylamide
Graphical Abstract
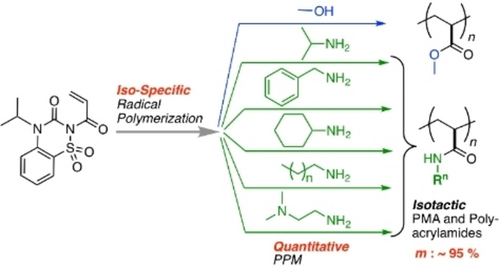
A pendant-transformable bulky acrylamide monomer was designed for the synthesis of a series of isotactic poly(alkyl acrylamide)s and poly(methyl acrylate). The stereospecific radical polymerization of the special monomer was controlled under diluted condition at lower temperature. The pendant underwent subsequent quantitative aminolysis and alcoholysis post-polymerization modification.
Abstract
We report syntheses of isotactic polyacrylate and polyacrylamide via a stereospecific radical polymerization of a pendant-transformable monomer, acrylamide carrying isopropyl-substituted ureidosulfonamide (1), followed by post-polymerization modification (PPM). The study in the alcoholysis and aminolysis reactions of the model compound (2) for evaluation of the transformation ability of the electron-withdrawing pendant group on the repeating unit 1 revealed the following points: the pendant of the polymer became more reactive than that of monomer; the pendant was active enough for aminolysis reaction affording the amide compound quantitatively without additive/catalyst; the addition of a lithium triflate [Li(OTf)] and triethylamine (Et3N) was effective as for promotion of the alcoholysis reaction. Poly(methyl acrylate) (PMA) was quantitatively obtained via the radical polymerization of 1 in the presence of Li(OTf) at 60 °C and the subsequent addition of methanol along with Et3N. Thus-obtained PMA showed higher isotacticity [m=74 %] than that directly obtained via radical polymerization of methyl acrylate (MA) (m=51 %). The isotacticity was further increased as the temperature and monomer concentration were lower, and eventually m was increased up to 93 %. The aminolysis PPM after the iso-specific radical polymerization of 1 gave various isotactic polyacrylamides carrying different alkyl pendant groups, including poly(N-isopropylacrylamide) (PNIPAM).
Open Research
Data Availability Statement
Research data are not shared.