Programmable Amine Synthesis via Iterative Boron Homologation
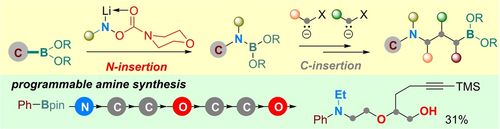
Graphical Abstract
Abstract
The value of Matteson-type reactions has been increasingly recognized for developing automated organic synthesis. However, the typical Matteson reactions almost exclusively focus on homologation of carbon units. Here, we report the detailed development of sequential insertion of nitrogen and carbon atoms into boronate C−B bonds, which provides a modular and iterative approach to access functionalized tertiary amines. A new class of nitrenoid reagents is uncovered to allow direct formation of aminoboranes from aryl or alkyl boronates via N-insertion. The one-pot N-insertion followed by controlled mono- or double-carbenoid insertion has been realized with widely available aryl boronates. The resulting aminoalkyl boronate products can undergo further homologation and various other transformations. Preliminary success on homologation of N,N-dialkylaminoboranes and sequential N- and C-insertions with alkyl boronates have also been achieved. To broaden the synthetic utility, selective removal of a benzyl or aryl substituent permits access to secondary or primary amine products. The application of this method has been demonstrated in the modular synthesis of bioactive compounds and the programmable construction of diamines and aminoethers. A plausible reaction mechanism, supported by preliminary NMR (nuclear magnetic resonance) and computational studies, is also proposed.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.