Enantioselective Synthesis of 1,2-Benzothiazine 1-Imines via RuII/Chiral Carboxylic Acid-Catalyzed C−H Alkylation/Cyclization
Long-Tao Huang
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorYuta Kitakawa
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorKodai Yamada
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorFuta Kamiyama
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorDr. Masahiro Kojima
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorCorresponding Author
Dr. Tatsuhiko Yoshino
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Shigeki Matsunaga
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorLong-Tao Huang
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorYuta Kitakawa
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorKodai Yamada
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorFuta Kamiyama
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorDr. Masahiro Kojima
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorCorresponding Author
Dr. Tatsuhiko Yoshino
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Shigeki Matsunaga
Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, 060-0812 Japan
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorGraphical Abstract
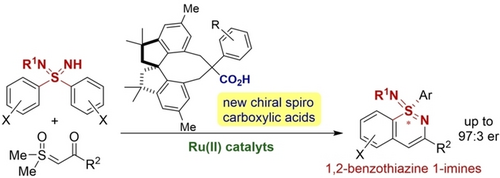
Enantioselective C−H alkylation/cyclization of sulfondiimines with sulfoxonium ylides using a RuII catalyst and a newly developed chiral spiro carboxylic acid enables the synthesis of 1,2-benzothiazine 1-imines, thus expanding the accessible chemical space of chiral hexavalent organosulfur scaffolds relevant to biologically active compounds.
Abstract
Sulfondiimines are diaza-analogues of sulfones with a chiral sulfur center. Compared to sulfones and sulfoximines, their synthesis and transformations have so far been studied to a lesser extent. Here, we report the enantioselective synthesis of 1,2-benzothiazine 1-imines, i.e., cyclic sulfondiimine derivatives from sulfondiimines and sulfoxonium ylides via C−H alkylation/cyclization reactions. The combination of [Ru(p-cymene)Cl2]2 and a newly developed chiral spiro carboxylic acid is key to achieving high enantioselectivity.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202305480-sup-0001-HLT-3ka.cif735.7 KB | Supporting Information |
| anie202305480-sup-0001-HLT-3ka.pdf75.1 KB | Supporting Information |
| anie202305480-sup-0001-misc_information.pdf9.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aE. A. Ilardi, E. Vitaku, J. T. Njardarson, J. Med. Chem. 2014, 57, 2832;
- 1bC. Zhao, K. P. Rakesh, L. Ravidar, W.-Y. Fang, H.-L. Qin, Eur. J. Med. Chem. 2019, 162, 679.
- 2
- 2aV. Bizet, C. M. M. Hendriks, C. Bolm, Chem. Soc. Rev. 2015, 44, 3378;
- 2bM. Frings, C. Bolm, A. Blum, C. Gnamm, Eur. J. Med. Chem. 2017, 126, 225;
- 2cJ. A. Bull, L. Degennaro, R. Luisi, Synlett 2017, 28, 2525;
- 2dP. Ghosh, B. Ganguly, S. Das, Asian J. Org. Chem. 2020, 9, 2035;
- 2eM. Andresini, A. Tota, L. Degennaro, J. A. Bull, R. Luisi, Chem. Eur. J. 2021, 27, 17293;
- 2fY. Han, K. Xing, J. Zhang, T. Tong, Y. Shi, H. Cao, H. Yu, Y. Zhang, D. Liu, L. Zhao, Eur. J. Med. Chem. 2021, 209, 112885.
- 3
- 3aM. T. Passia, J.-H. Schöbel, C. Bolm, Chem. Soc. Rev. 2022, 51, 4890. For the synthesis of sulfondiimines in this work, see:
- 3bT. Yoshimura, H. Ishikawa, T. Fujie, E. Takata, R. Miyatake, H. Kita, E. Tsukurimichi, Synthesis 2008, 1835;
- 3cM. Candy, C. Guyon, S. Mersmann, J.-R. Chen, C. Bolm, Angew. Chem. Int. Ed. 2012, 51, 4440, and references therein.
- 4For selected reviews on C−H functionalization, see:
- 4aC. G. Newton, S.-G. Wang, C. C. Oliveira, N. Cramer, Chem. Rev. 2017, 117, 8908;
- 4bJ. R. Hummel, J. A. Boerth, J. A. Ellman, Chem. Rev. 2017, 117, 9163;
- 4cZ. Dong, Z. Ren, S. J. Thompson, Y. Xu, G. Dong, Chem. Rev. 2017, 117, 9333;
- 4dT. G. Saint-Denis, R.-Y. Zhu, G. Chen, Q.-F. Wu, J.-Q. Yu, Science 2018, 359, eaao4798;
- 4eC. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes, M. Schnürch, Chem. Soc. Rev. 2018, 47, 6603;
- 4fJ. Loup, U. Dhawa, F. Pesciaioli, J. Wencel-Delord, L. Ackermann, Angew. Chem. Int. Ed. 2019, 58, 12803;
- 4gJ. Mas-Roselló, A. G. Herraiz, B. Audic, A. Laverny, N. Cramer, Angew. Chem. Int. Ed. 2021, 60, 13198;
- 4hT. Dalton, T. Faber, F. Glorius, ACS Cent. Sci. 2021, 7, 245;
- 4iU. Dutta, S. Maiti, T. Bhattacharya, D. Maiti, Science 2021, 372, eabd5992;
- 4jT. Rogge, N. Kaplaneris, N. Chatani, J. Kim, S. Chang, B. Punji, L. L. Schafer, D. G. Musaev, J. Wencel-Delord, C. A. Roberts, R. Sarpong, Z. E. Wilson, M. A. Brimble, M. J. Johansson, L. Ackermann, Nat. Rev. Methods Primers 2021, 1, 43;
- 4kQ. Zhang, L.-S. Wu, B.-F. Shi, Chem 2022, 8, 384.
- 5For seminal and selected examples, see:
- 5aW. Dong, L. Wang, K. Parthasarathy, F. Pan, C. Bolm, Angew. Chem. Int. Ed. 2013, 52, 11573;
- 5bD.-G. Yu, F. de Azambuja, F. Glorius, Angew. Chem. Int. Ed. 2014, 53, 2754;
- 5cK. Parthasarathy, C. Bolm, Chem. Eur. J. 2014, 20, 4896;
- 5dW. Dong, K. Parthasarathy, Y. Cheng, F. Pan, C. Bolm, Chem. Eur. J. 2014, 20, 15732;
- 5eY. Cheng, C. Bolm, Angew. Chem. Int. Ed. 2015, 54, 12349;
- 5fJ. Huang, Y. Huang, T. Wang, Q. Huang, Z. Wang, Z. Chen, Org. Lett. 2017, 19, 1128;
- 5gY. N. Aher, D. M. Lade, A. B. Pawar, Chem. Commun. 2018, 54, 6288;
- 5hH. Xie, J. Lan, J. Gui, F. Chen, H. Jiang, W. Zeng, Adv. Synth. Catal. 2018, 360, 3534;
- 5iS. Li, L. Liu, R. Wang, Y. Yang, J. Li, J. Wei, Org. Lett. 2020, 22, 7470;
- 5jP. Shi, Y. Tu, C. Wang, D. Kong, D. Ma, C. Bolm, Org. Lett. 2020, 22, 8842;
- 5kH.-B. Xu, J.-H. Yang, X.-Y. Chai, Y.-Y. Zhu, L. Dong, Org. Lett. 2020, 22, 2060.
- 6For enantioselective variants, see:
- 6aB. Shen, B. Wan, X. Li, Angew. Chem. Int. Ed. 2018, 57, 15534;
- 6bY. Sun, N. Cramer, Angew. Chem. Int. Ed. 2018, 57, 15539;
- 6cM. Brauns, N. Cramer, Angew. Chem. Int. Ed. 2019, 58, 8902;
- 6dT. Zhou, P.-F. Qian, J.-Y. Li, Y.-B. Zhou, H.-C. Li, H.-Y. Chen, B.-F. Shi, J. Am. Chem. Soc. 2021, 143, 6810;
- 6eK. Mukherjee, N. Grimblat, S. Sau, K. Ghosh, M. Shankar, V. Gandon, A. K. Sahoo, Chem. Sci. 2021, 12, 14863;
- 6fL.-T. Huang, Y. Hirata, Y. Kato, L. Lin, M. Kojima, T. Yoshino, S. Matsunaga, Synthesis 2022, 54, 4703;
- 6gY. Hirata, D. Sekine, Y. Kato, L. Lin, M. Kojima, T. Yoshino, S. Matsunaga, Angew. Chem. Int. Ed. 2022, 61, e202205341;
- 6hJ.-Y. Li, P.-P. Xie, T. Zhou, P.-F. Qian, Y.-B. Zhou, H.-C. Li, X. Hong, B.-F. Shi, ACS Catal. 2022, 12, 9083;
- 6iY.-B. Zhou, T. Zhou, P.-F. Qian, J.-Y. Li, B.-F. Shi, ACS Catal. 2022, 12, 9806;
- 6jP.-F. Qian, T. Zhou, J.-Y. Li, Y.-B. Zhou, B.-F. Shi, ACS Catal. 2022, 12, 13876;
- 6kS.-Y. Song, X. Zhou, Z. Ke, S. Xu, Angew. Chem. Int. Ed. 2023, 62, e202217130.
- 7R. A. Bohmann, J.-H. Schöbel, Y. Unoh, M. Miura, C. Bolm, Adv. Synth. Catal. 2019, 361, 2000.
- 8
- 8aR. Bentley, Chem. Soc. Rev. 2005, 34, 609;
- 8bW. Liu, J. Ke, C. He, Chem. Sci. 2021, 12, 10972;
- 8cP.-F. Qian, J.-Y. Li, T. Zhou, B.-F. Shi, Synthesis 2022, 54, 4784.
- 9For seminal work and selected reviews on RuII-catalyzed directed C−H functionalization, see:
- 9aS. Oi, S. Fukita, N. Hirata, N. Watanuki, S. Miyano, Y. Inoue, Org. Lett. 2001, 3, 2579;
- 9bP. B. Arockiam, C. Bruneau, P. H. Dixneuf, Chem. Rev. 2012, 112, 5879;
- 9cL. Ackermann, Acc. Chem. Res. 2014, 47, 281;
- 9dS. De Sarkar, W. Liu, S. I. Kozhushkov, L. Ackermann, Adv. Synth. Catal. 2014, 356, 1461;
- 9eJ. A. Leitch, C. G. Frost, Chem. Soc. Rev. 2017, 46, 7145;
- 9fC. Shan, L. Zhu, L.-B. Qu, R. Bai, Y. Lan, Chem. Soc. Rev. 2018, 47, 7552.
- 10For examples on RuII-catalyzed directed enantioselective C−H functionalization, see:
- 10aZ.-Y. Li, H. H. C. Lakmal, X. Qian, Z. Zhu, B. Donnadieu, S. J. McClain, X. Xu, X. Cui, J. Am. Chem. Soc. 2019, 141, 15730;
- 10bG. Li, Q. Liu, L. Vasamsetty, W. Guo, J. Wang, Angew. Chem. Int. Ed. 2020, 59, 3475;
- 10cU. Dhawa, R. Connon, J. C. A. Oliveira, R. Steinbock, L. Ackermann, Org. Lett. 2021, 23, 2760;
- 10dY. Li, Y.-C. Liou, J. C. A. Oliveira, L. Ackermann, Angew. Chem. Int. Ed. 2022, 61, e202212595;
- 10eH. Liang, W. Guo, J. Li, J. Jiang, J. Wang, Angew. Chem. Int. Ed. 2022, 61, e202204926. See also refs. [6d,f], and [6j]. For a recent review, see:
- 10fH. Liang, J. Wang, Chem. Eur. J. 2023, 29, e202202461.
- 11For carboxylate-assisted C−H activation, see:
- 11aD. Lapointe, K. Fagnou, Chem. Lett. 2010, 39, 1118;
- 11bL. Ackermann, Chem. Rev. 2011, 111, 1315;
- 11cD. L. Davies, S. A. Macgregor, C. L. McMullin, Chem. Rev. 2017, 117, 8649;
- 11dT. Rogge, J. C. A. Oliveira, R. Kuniyil, L. Hu, L. Ackermann, ACS Catal. 2020, 10, 10551.
- 12For chiral ligand-assisted Pd-catalyzed enantioselective C−H activation, see:
- 12aB.-F. Shi, N. Maugel, Y.-H. Zhang, J.-Q. Yu, Angew. Chem. Int. Ed. 2008, 47, 4882;
- 12bY.-F. Yang, X. Hong, J.-Q. Yu, K. N. Houk, Acc. Chem. Res. 2017, 50, 2853;
- 12cQ. Shao, K. Wu, Z. Zhuang, S. Qian, J.-Q. Yu, Acc. Chem. Res. 2020, 53, 833;
- 12dE. L. Lucas, N. Y. S. Lam, Z. Zhuang, H. S. S. Chan, D. A. Strassfeld, J.-Q. Yu, Acc. Chem. Res. 2022, 55, 537, and references therein.
- 13For seminal work and reviews on chiral carboxylic acid-assisted group 9 metal-catalyzed enantioselective C−H activation, see
- 13aD. Gwon, S. Park, S. Chang, Tetrahedron 2015, 71, 4504;
- 13bL. Lin, S. Fukagawa, D. Sekine, E. Tomita, T. Yoshino, S. Matsunaga, Angew. Chem. Int. Ed. 2018, 57, 12048;
- 13cT. Yoshino, S. Matsunaga, ACS Catal. 2021, 11, 6455;
- 13dT. Yoshino, Bull. Chem. Soc. Jpn. 2022, 95, 1280.
- 14
- 14aS. Fukagawa, Y. Kato, R. Tanaka, M. Kojima, T. Yoshino, S. Matsunaga, Angew. Chem. Int. Ed. 2019, 58, 1153;
- 14bD. Sekine, K. Ikeda, S. Fukagawa, M. Kojima, T. Yoshino, S. Matsunaga, Organometallics 2019, 38, 3921;
- 14cS. Fukagawa, M. Kojima, T. Yoshino, S. Matsunaga, Angew. Chem. Int. Ed. 2019, 58, 18154;
- 14dY. Kato, L. Lin, M. Kojima, T. Yoshino, S. Matsunaga, ACS Catal. 2021, 11, 4271.
- 15
- 15aS. Chang, L. Wang, X. Lin, Org. Biomol. Chem. 2018, 16, 2239;
- 15bH. Gu, Z. Han, H. Xie, X. Lin, Org. Lett. 2018, 20, 6544;
- 15cH. Shan, Q. Zhou, J. Yu, S. Zhang, X. Hong, X. Lin, J. Org. Chem. 2018, 83, 11873;
- 15dW. Sun, H. Gu, X. Lin, J. Org. Chem. 2018, 83, 4034;
- 15eL. Wang, J. Zhong, X. Lin, Angew. Chem. Int. Ed. 2019, 58, 15824;
- 15fZ. Han, X. Lin, Synthesis 2020, 52, 1131;
- 15gZ. Han, J. Jin, A. G. Woldegiorgis, X. Lin, RSC Adv. 2022, 12, 27012;
- 15hL. Yao, A. G. Woldegiorgis, X. Lin, Tetrahedron Lett. 2022, 109, 154157.
- 16For the calculated structures of L4 and L8, see the Supporting Information.
- 17The absolute configuration of 3 ka was determined via single-crystal X-ray diffraction analysis. Deposition Number 2257084 (for 3 ka) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 18The absolute configuration of the major isomer of the recovered sulfondiimine (5) was determined by the comparison of its retention time in chiral HPLC analysis and specific rotation with those were previously reported: S. Dong, M. Frings, H. Cheng, J. Wen, D. Zhang, G. Raabe, C. Bolm, J. Am. Chem. Soc. 2016, 138, 2166, and ref. [3c]. See the Supporting Information for details.
- 19For preliminary results of a parallel kinetic resolution of an unsymmetrically substituted diaryl sulfondiimine, see the Supporting Information.
- 20C. S. Chen, Y. Fujimoto, G. Girdaukas, C. J. Sih, J. Am. Chem. Soc. 1982, 104, 7294.