Bifunctional NHC-Catalyzed Remote Enantioselective Mannich-type Reaction of 5-(Chloromethyl)furfural via Trienolate Intermediates
Dr. Yuan-Yuan Gao
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
Henan Engineering Laboratory of Green Synthesis for Pharmaceuticals, College of Chemistry and Chemical Engineering, Shangqiu Normal University, Shangqiu, 476000 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Chun-Lin Zhang
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
These authors contributed equally to this work.
Search for more papers by this authorMing-Lei Jin
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Dr. Zhong-Hua Gao
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Song Ye
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorDr. Yuan-Yuan Gao
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
Henan Engineering Laboratory of Green Synthesis for Pharmaceuticals, College of Chemistry and Chemical Engineering, Shangqiu Normal University, Shangqiu, 476000 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Chun-Lin Zhang
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
These authors contributed equally to this work.
Search for more papers by this authorMing-Lei Jin
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Dr. Zhong-Hua Gao
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Song Ye
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorGraphical Abstract
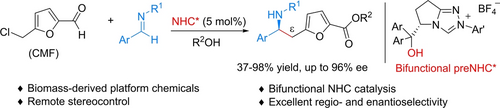
NHC-catalyzed enantioselective Mannich-type reactions of 5-(chloromethyl)furfural (CMF), an important biomass-derived platform chemical, with aldimines afford chiral amines in good yields with excellent regio- and enantioselectivities. The use of a bifunctional NHC bearing a free hydroxy group enabled the remote addition of the trienolate intermediate to the imine to occur in a highly stereocontrolled manner.
Abstract
N-heterocyclic carbene (NHC)-catalyzed enantioselective Mannich-type reactions of the biomass-derived platform compound 5-(chloromethyl)furfural (CMF) with imines were developed. A series of high-value-added chiral amines were afforded in good to high yields with excellent regio- and enantioselectivities. The bifunctional NHC derived from ʟ-pyroglutamic acid efficiently steered the remote addition of the trienolate intermediate to the imine in a highly stereocontrolled manner. This represents the first enantioselective reaction proceeding via an NHC-bound trienolate intermediate.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202301126-sup-0001-misc_information.pdf17.9 MB | Supporting Information |
| anie202301126-sup-0001-MX9226.cif474.2 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. J. Bozell, Science 2010, 329, 522;
- 1bH. Kopetz, Nature 2013, 494, 29;
- 1cY. Queneau, B. Han, The Innovation 2022, 3, 100184.
- 2
- 2aC.-H. Zhou, X. Xia, C.-X. Lin, D.-S. Tong, J. Beltramini, Chem. Soc. Rev. 2011, 40, 5588;
- 2bR. Rinaldi, Angew. Chem. Int. Ed. 2014, 53, 8559;
- 2cZ. Zhang, J. Song, B. Han, Chem. Rev. 2017, 117, 6834.
- 3
- 3aR.-J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres, J. G. de Vries, Chem. Rev. 2013, 113, 1499;
- 3bJ. Wang, J. Xi, Y. Wang, Green Chem. 2015, 17, 737;
- 3cZ. Zhang, K. Deng, ACS Catal. 2015, 5, 6529;
- 3dM. Mascal, ChemSusChem 2015, 8, 3391;
- 3eS. Chen, R. Wojcieszak, F. Dumeignil, E. Marceau, S. Royer, Chem. Rev. 2018, 118, 11023;
- 3fM. Mascal, ACS Sustainable Chem. Eng. 2019, 7, 5588;
- 3gB. Wozniak, S. Tin, J. G. de Vries, Chem. Sci. 2019, 10, 6024.
- 4
- 4aA. Corma, S. Iborra, A. Velty, Chem. Rev. 2007, 107, 2411;
- 4bC. O. Tuck, E. Pérez, I. T. Horváth, R. A. Sheldon, M. Poliakoff, Science 2012, 337, 695;
- 4cM. Besson, P. Gallezot, C. Pinel, Chem. Rev. 2014, 114, 1827;
- 4dL. Wu, T. Moteki, A. A. Gokhale, D. W. Flaherty, F. D. Toste, Chem 2016, 1, 32;
- 4eT. A. Bender, J. A. Dabrowski, M. R. Gagné, Nat. Chem. Rev. 2018, 2, 35.
- 5
- 5aD. Enders, O. Niemeier, A. Henseler, Chem. Rev. 2007, 107, 5606;
- 5bS. De Sarkar, A. Biswas, R. C. Samanta, A. Studer, Chem. Eur. J. 2013, 19, 4664;
- 5cS. J. Ryan, L. Candish, D. W. Lupton, Chem. Soc. Rev. 2013, 42, 4906;
- 5dM. N. Hopkinson, C. Richter, M. Schedler, F. Glorius, Nature 2014, 510, 485;
- 5eD. M. Flanigan, F. Romanov-Michailidis, N. A. White, T. Rovis, Chem. Rev. 2015, 115, 9307;
- 5fM. H. Wang, K. A. Scheidt, Angew. Chem. Int. Ed. 2016, 55, 14912;
- 5gK. J. R. Murauski, A. A. Jaworski, K. A. Scheidt, Chem. Soc. Rev. 2018, 47, 1773;
- 5hP. Bellotti, M. Koy, M. N. Hopkinson, F. Glorius, Nat. Chem. Rev. 2021, 5, 711.
- 6
- 6aJ. C. Sheehan, D. H. Hunneman, J. Am. Chem. Soc. 1966, 88, 3666;
- 6bD. Enders, U. Kallfass, Angew. Chem. Int. Ed. 2002, 41, 1743;
10.1002/1521-3773(20020517)41:10<1743::AID-ANIE1743>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 6cS. M. Mennen, J. D. Gipson, Y. R. Kim, S. J. Miller, J. Am. Chem. Soc. 2005, 127, 1654;
- 6dJ. Read de Alaniz, T. Rovis, J. Am. Chem. Soc. 2005, 127, 6284;
- 6eH. Takikawa, Y. Hachisu, J. W. Bode, K. Suzuki, Angew. Chem. Int. Ed. 2006, 45, 3492;
- 6fX. Huang, S. Ye, Chin. Sci. Bull. 2010, 55, 1753;
- 6gI. Piel, M. Steinmetz, K. Hirano, R. Fröhlich, S. Grimme, F. Glorius, Angew. Chem. Int. Ed. 2011, 50, 4983;
- 6hL.-H. Sun, Z.-Q. Liang, W.-Q. Jia, S. Ye, Angew. Chem. Int. Ed. 2013, 52, 5803;
- 6iM.-Q. Jia, S.-L. You, ACS Catal. 2013, 3, 622.
- 7
- 7aM. He, J. R. Struble, J. W. Bode, J. Am. Chem. Soc. 2006, 128, 8418;
- 7bY.-R. Zhang, L. He, X. Wu, P.-L. Shao, S. Ye, Org. Lett. 2008, 10, 277;
- 7cN. Duguet, C. D. Campbell, A. M. Z. Slawin, A. D. Smith, Org. Biomol. Chem. 2008, 6, 1108;
- 7dX. Zhao, K. E. Ruhl, T. Rovis, Angew. Chem. Int. Ed. 2012, 51, 12330;
- 7eL. Hao, Y. Du, H. Lv, X. Chen, H. Jiang, Y. Shao, Y. R. Chi, Org. Lett. 2012, 14, 2154.
- 8
- 8aC. Burstein, F. Glorius, Angew. Chem. Int. Ed. 2004, 43, 6205;
- 8bS. S. Sohn, E. L. Rosen, J. W. Bode, J. Am. Chem. Soc. 2004, 126, 14370;
- 8cA. Chan, K. A. Scheidt, J. Am. Chem. Soc. 2008, 130, 2740;
- 8dS. De Sarkar, A. Studer, Angew. Chem. Int. Ed. 2010, 49, 9266;
- 8eF.-G. Sun, L.-H. Sun, S. Ye, Adv. Synth. Catal. 2011, 353, 3134;
- 8fL. Candish, D. W. Lupton, J. Am. Chem. Soc. 2013, 135, 58;
- 8gJ. Cheng, Z. Huang, Y. R. Chi, Angew. Chem. Int. Ed. 2013, 52, 8592;
- 8hX.-Y. Chen, Z.-H. Gao, C.-Y. Song, C.-L. Zhang, Z.-X. Wang, S. Ye, Angew. Chem. Int. Ed. 2014, 53, 11611.
- 9
- 9aX.-Y. Chen, Q. Liu, P. Chauhan, D. Enders, Angew. Chem. Int. Ed. 2018, 57, 3862;
- 9bJ. Gao, J. Feng, D. Du, Org. Chem. Front. 2021, 8, 6138.
- 10L.-T. Shen, P.-L. Shao, S. Ye, Adv. Synth. Catal. 2011, 353, 1943.
- 11J. Mo, X. Chen, Y. R. Chi, J. Am. Chem. Soc. 2012, 134, 8810.
- 12
- 12aX.-Y. Chen, F. Xia, J.-T. Cheng, S. Ye, Angew. Chem. Int. Ed. 2013, 52, 10644;
- 12bZ. Xiao, C. Yu, T. Li, X.-S. Wang, C. Yao, Org. Lett. 2014, 16, 3632;
- 12cB.-S. Li, Y. Wang, Z. Jin, P. Zheng, R. Ganguly, Y. R. Chi, Nat. Commun. 2015, 6, 6207;
- 12dZ. Wu, F. Li, J. Wang, Angew. Chem. Int. Ed. 2015, 54, 1629.
- 13
- 13aX. Chen, S. Yang, B.-A. Song, Y. R. Chi, Angew. Chem. Int. Ed. 2013, 52, 11134;
- 13bD. Janssen-Müller, S. Singha, T. Olyschläger, C. G. Daniliuc, F. Glorius, Org. Lett. 2016, 18, 4444;
- 13cD.-F. Chen, T. Rovis, Synthesis 2017, 49, 293;
- 13dA. Przydacz, A. Topolska, A. Skrzyńska, Ł. Albrecht, Adv. Synth. Catal. 2022, 364, 1434.
- 14
- 14aM. Kowalczyk, D. W. Lupton, Angew. Chem. Int. Ed. 2014, 53, 5314;
- 14bR. M. Gillard, J. E. M. Fernando, D. W. Lupton, Angew. Chem. Int. Ed. 2018, 57, 4712.
- 15T. Zhu, C. Mou, B. Li, M. Smetankova, B.-A. Song, Y. R. Chi, J. Am. Chem. Soc. 2015, 137, 5658.
- 16
- 16aK. Xu, W. Li, S. Zhu, T. Zhu, Angew. Chem. Int. Ed. 2019, 58, 17625;
- 16bK. Xu, Z. Wang, T. Zhu, Synlett 2020, 31, 925.
- 17
- 17aL. Dai, Z.-H. Xia, Y.-Y. Gao, Z.-H. Gao, S. Ye, Angew. Chem. Int. Ed. 2019, 58, 18124;
- 17bY.-Y. Xu, L. Dai, Z.-H. Gao, S. Ye, J. Org. Chem. 2022, 87, 14970.
- 18L. Dai, S. Ye, ACS Catal. 2020, 10, 994.
- 19L. Dai, Y. Qiu, Y.-Y. Xu, S. Ye, Cell Rep. Phys. Sci. 2020, 1, 100071.
- 20
- 20aL. Bernardi, J. López-Cantarero, B. Niess, K. A. Jørgensen, J. Am. Chem. Soc. 2007, 129, 5772;
- 20bD. Uraguchi, K. Yoshioka, Y. Ueki, T. Ooi, J. Am. Chem. Soc. 2012, 134, 19370;
- 20cL. Dell'Amico, Ł. Albrecht, T. Naicker, P. H. Poulsen, K. A. Jørgensen, J. Am. Chem. Soc. 2013, 135, 8063;
- 20dR. Deng, S. Wu, C. Mou, J. Liu, P. Zheng, X. Zhang, Y. R. Chi, J. Am. Chem. Soc. 2022, 144, 5441.
- 21
- 21aA. Przydacz, A. Skrzyńska, Ł. Albrecht, Angew. Chem. Int. Ed. 2019, 58, 63;
- 21bB.-X. Xiao, X.-Y. Gao, W. Du, Y.-C. Chen, Chem. Eur. J. 2019, 25, 1607.
- 22
- 22aJ. M. M. Verkade, L. J. C. v. Hemert, P. J. L. M. Quaedflieg, F. P. J. T. Rutjes, Chem. Soc. Rev. 2008, 37, 29;
- 22bA. Ting, S. E. Schaus, Eur. J. Org. Chem. 2007, 5797;
- 22cB. Karimi, D. Enders, E. Jafari, Synthesis 2013, 45, 2769;
- 22dM. S. Roselló, C. del Pozo, S. Fustero, Synthesis 2016, 48, 2553;
- 22eS. Saranya, N. A. Harry, K. K. Krishnan, G. Anilkumar, Asian J. Org. Chem. 2018, 7, 613;
- 22fI. Bagheri, L. Mohammadi, V. Zadsirjan, M. M. Heravi, ChemistrySelect 2021, 6, 1008.
- 23X.-Y. Chen, Z.-H. Gao, S. Ye, Acc. Chem. Res. 2020, 53, 690.
- 24C. Zhao, F. Li, J. Wang, Angew. Chem. Int. Ed. 2016, 55, 1820.
- 25M. Wadamoto, E. M. Phillips, T. E. Reynolds, K. A. Scheidt, J. Am. Chem. Soc. 2007, 129, 10098.
- 26L. He, Y.-R. Zhang, X.-L. Huang, S. Ye, Synthesis 2008, 2825.
- 27Deposition number 2237090 (3 a) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.