Enantioselective Synthesis of N−N Bisindole Atropisomers
Peng Zhang
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
Search for more papers by this authorQi Xu
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
Search for more papers by this authorXiao-Mei Wang
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
Search for more papers by this authorDr. Jia Feng
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
Search for more papers by this authorDr. Chuan-Jun Lu
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
Search for more papers by this authorCorresponding Author
Dr. Yingzi Li
Shenzhen Key Laboratory for the Intelligent Microbial Manufacturing of Medicines, Shenzhen Institute of Synthetic Biology, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Ren-Rong Liu
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
Search for more papers by this authorPeng Zhang
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
Search for more papers by this authorQi Xu
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
Search for more papers by this authorXiao-Mei Wang
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
Search for more papers by this authorDr. Jia Feng
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
Search for more papers by this authorDr. Chuan-Jun Lu
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
Search for more papers by this authorCorresponding Author
Dr. Yingzi Li
Shenzhen Key Laboratory for the Intelligent Microbial Manufacturing of Medicines, Shenzhen Institute of Synthetic Biology, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Ren-Rong Liu
College of Chemistry and Chemical Engineering, Qingdao University, Ningxia Road 308#, Qingdao, 266071 China
Search for more papers by this authorGraphical Abstract
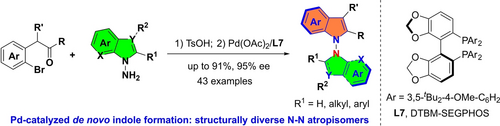
An enantioselective synthesis of N−N bisindole atropisomers based on the de novo construction of one indole skeleton is presented. A wide variety of N−N axially chiral bisindoles were obtained in good yields with excellent enantioselectivities. Structurally diverse indole-pyrrole, indole-carbazole and non-biaryl-indole atropisomers possessing a chiral N−N axis were accessed using this protocol.
Abstract
N−N Atropisomers are a common motif in natural products and represent a significant dimension for exploration in modern pharmaceutical and medicinal chemistry. However, the catalytic atroposelective synthesis of such molecules remains challenging, hampering meaningful development. In particular, an enantioselective synthesis of N−N bisindole atropisomers is unprecedented. Herein, the first enantioselective synthesis of N−N bisindole atropisomers via the palladium-catalyzed de novo construction of one indole skeleton is presented. A wide variety of N−N axially chiral bisindoles were generated in good yields with excellent enantioselectivities via a cascade condensation/N-arylation reaction. Structurally diverse indole-pyrrole, indole-carbazole, and non-biaryl-indole atropisomers possessing a chiral N−N axis were accessed using this protocol. Moreover, investigations using density functional theory (DFT) calculations provided insight into the reaction mechanism and enantiocontrol.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202212101-sup-0001-misc_information.pdf19.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aL. M. Blair, J. Sperry, J. Nat. Prod. 2013, 76, 794–812;
- 1bP. S. Reddy, A. K. D. Bhavani, Adv. Heterocycl. Chem. 2015, 114, 271–391;
- 1cG.-J. Mei, W. L. Koay, C.-Y. Guan, Y. Lu, Chem 2022, https://doi.org/10.1016/j.chempr.2022.04.011.
- 2
- 2aQ. Zhang, A. Mándi, S. Li, Y. Chen, W. Zhang, X. Tian, H. Zhang, H. Li, W. Zhang, S. Zhang, J. Ju, T. Kurtán, C. Zhang, Eur. J. Org. Chem. 2012, 5256–5262;
- 2bZ. Xu, M. Baunach, L. Ding, C. Hertweck, Angew. Chem. Int. Ed. 2012, 51, 10293–10297; Angew. Chem. 2012, 124, 10439–10443;
- 2cM. Shoeb, S. Celik, M. Jaspars, Y. Kumarasamy, S. M. MacManus, L. Nahar, P. K. Thoo-Lin, S. D. Sarker, Tetrahedron 2005, 61, 9001–9006;
- 2dK. Suzuki, I. Nomura, M. Ninomiya, K. Tanaka, M. Koketsu, Bioorg. Med. Chem. Lett. 2018, 28, 2976–2978.
- 3
- 3aJ. Wencel-Delord, A. Panossian, F. R. Leroux, F. Colobert, Chem. Soc. Rev. 2015, 44, 3418–3430;
- 3bA. Link, C. Sparr, Chem. Soc. Rev. 2018, 47, 3804–3815;
- 3cO. Kitagawa, Acc. Chem. Res. 2021, 54, 719–730;
- 3dF. Colobert, B.-F. Shi, Chem. Catal. 2021, 1, 483–485;
- 3eJ. K. Cheng, S.-H. Xiang, S. Li, L. Ye, B. Tan, Chem. Rev. 2021, 121, 4805–4902.
- 4C. Chang, R. Adams, J. Am. Chem. Soc. 1931, 53, 2353–2357.
- 5G.-J. Mei, J. J. Wong, W. Zheng, A. A. Nangia, K. N. Houk, Y. Lu, Chem 2021, 7, 2743–2757.
- 6
- 6aW. Lin, Q. Zhao, Y. Li, M. Pan, C. Yang, G. Yang, X. Li, Chem. Sci. 2022, 13, 141–148;
- 6bM. Pan, Y.-B. Shao, Q. Zhao, X. Li, Org. Lett. 2022, 24, 374–378.
- 7K.-W. Chen, Z.-H. Chen, S. Yang, S.-F. Wu, Y.-C. Zhang, F. Shi, Angew. Chem. Int. Ed. 2022, 61, e202116829; Angew. Chem. 2022, 134, e202116829.
- 8Y. Gao, L.-Y. Wang, T. Zhang, B.-M. Yang, Y. Zhao, Angew. Chem. Int. Ed. 2022, 61, e202200371; Angew. Chem. 2022, 134, e202200371.
- 9
- 9aX.-M. Wang, P. Zhang, Q. Xu, C.-Q. Guo, D.-B. Zhang, C.-J. Lu, R.-R. Liu, J. Am. Chem. Soc. 2021, 143, 15005–15010;
- 9bQ. Xu, H. Zhang, F.-B. Ge, X.-M. Wang, P. Zhang, C.-J. Lu, R.-R. Liu, Org. Lett. 2022, 24, 3138–3143.
- 10
- 10aT. Z. Li, S. J. Liu, W. Tan, F. Shi, Chem. Eur. J. 2020, 26, 15779–15792;
- 10bY.-C. Zhang, F. Jiang, F. Shi, Acc. Chem. Res. 2020, 53, 425–446.
- 11
- 11aC. He, M. Hou, Z. Zhu, Z. Gu, ACS Catal. 2017, 7, 5316–5320;
- 11bC. Ma, F. Jiang, F.-T. Sheng, Y. Jiao, G.-J. Mei, F. Shi, Angew. Chem. Int. Ed. 2019, 58, 3014–3020; Angew. Chem. 2019, 131, 3046–3052;
- 11cF. Jiang, K.-W. Chen, P. Wu, Y.-C. Zhang, Y. Jiao, F. Shi, Angew. Chem. Int. Ed. 2019, 58, 15104–15110; Angew. Chem. 2019, 131, 15248–15254;
- 11dW. Xia, Q.-J. An, S.-H. Xiang, S. Li, Y.-B. Wang, B. Tan, Angew. Chem. Int. Ed. 2020, 59, 6775–6779; Angew. Chem. 2020, 132, 6841–6845;
- 11eA. Kim, A. Kim, S. Park, S. Kim, H. Jo, K. M. Ok, S. K. Lee, J. Song, Y. Kwon, Angew. Chem. Int. Ed. 2021, 60, 12279–12283; Angew. Chem. 2021, 133, 12387–12391;
- 11fH. Chen, X. Zhou, N. Li, D. Ji, F. Wang, Y. Lan, X. Li, Angew. Chem. Int. Ed. 2022, 61, e202111860; Angew. Chem. 2022, 134, e202111860;
- 11gJ. Frey, A. Malekafzali, I. Delso, S. Choppin, F. Colobert, J. Wencel-Delord, Angew. Chem. Int. Ed. 2020, 59, 8844–8848; Angew. Chem. 2020, 132, 8929–8933;
- 11hJ. Frey, X. Hou, L. Ackermann, Chem. Sci. 2022, 13, 2729–2734.
- 12
- 12aN. Ototake, Y. Morimoto, A. Mokuya, H. Fukaya, Y. Shida, O. Kitagawa, Chem. Eur. J. 2010, 16, 6752–6755;
- 12bY. Morimoto, S. Shimizu, A. Mokuya, N. Ototake, A. Saito, O. Kitagawa, Tetrahedron 2016, 72, 5221–5229.
- 13M. Tian, D. Bai, G. Zheng, J. Chang, X. Li, J. Am. Chem. Soc. 2019, 141, 9527–9532.
- 14
- 14aY.-P. He, H. Wu, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2020, 59, 2105–2109; Angew. Chem. 2020, 132, 2121–2125;
- 14bX. Li, L. Zhao, Z. Qi, X. Li, Org. Lett. 2021, 23, 5901–5905;
- 14cC.-S. Wang, L. Wei, C. Fu, X.-H. Wang, C.-J. Wang, Org. Lett. 2021, 23, 7401–7406.
- 15L. Sun, H. Chen, B. Liu, J. Chang, L. Kong, F. Wang, Y. Lan, X. Li, Angew. Chem. Int. Ed. 2021, 60, 8391–8395; Angew. Chem. 2021, 133, 8472–8476.
- 16For a palladium catalyzed synthesis of 1-aminoindole derivatives, see:
- 16aM. Watanabe, T. Yamamoto, M. Nishiyama, Angew. Chem. Int. Ed. 2000, 39, 2501–2504;
10.1002/1521-3773(20000717)39:14<2501::AID-ANIE2501>3.0.CO;2-T CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 2620–2623; for indole synthesis reviews, see:
- 16bS. Cacchi, G. Fabrizi, Chem. Rev. 2011, 111, PR215–PR283;
- 16cP. Ruiz-Castillo, S. L. Buchwald, Chem. Rev. 2016, 116, 12564–12649.
- 17
- 17aI. Takahashia, F. Moritaa, S. Kusagayaa, H. Fukayab, O. Kitagawa, Tetrahedron: Asymmetry 2012, 23, 1657–1662;
- 17bH. Li, K. M. Belyk, J. Yin, Q. Chen, A. Hyde, Y. Ji, S. Oliver, M. T. Tudge, L. C. Campeau, K. R. Campos, J. Am. Chem. Soc. 2015, 137, 13728–13731.
- 18E. Picazo, S. M. Anthony, M. Giroud, A. Simon, M. A. Miller, K. N. Houk, N. K. Garg, J. Am. Chem. Soc. 2018, 140, 7605–7610.
- 19Deposition numbers 2177437 (3 a) and 2177438 (4 k) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 20
- 20aY. Sunesson, E. Limé, S. O. Nilsson Lill, R. E. Meadows, P.-O. Norrby, J. Org. Chem. 2014, 79, 11961–11969;
- 20bS.-T. Kim, B. Pudasaini, M.-H. Baik, ACS Catal. 2019, 9, 6851–6856.