Enantioselective [2+2] Cycloaddition of Allenyl Imide with Mono- or Disubstituted Alkenes
Wanlong Xiao
Key laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorLichao Ning
Key laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorShuang Xin
Key laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorProf. Dr. Shunxi Dong
Key laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaohua Liu
Key laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaoming Feng
Key laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorWanlong Xiao
Key laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorLichao Ning
Key laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorShuang Xin
Key laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorProf. Dr. Shunxi Dong
Key laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaohua Liu
Key laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaoming Feng
Key laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorGraphical Abstract
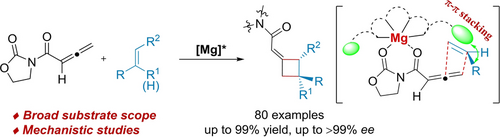
An efficient catalytic asymmetric [2+2] cycloaddition reaction of allenyl imide and alkenes was achieved by utilizing chiral N,N′-dioxide-magnesium(II) complex as the catalyst. This protocol provided a series of axially chiral cyclobutenes in high yields with excellent enantioselectivities. A stepwise mechanism was proposed based on experimental studies and DFT calculations and π–π stacking interaction was crucial for the enantioselectivity.
Abstract
An efficient catalytic asymmetric [2+2] cycloaddition of allenyl imide and mono- or disubstituted alkenes is disclosed. The key feature of this method is the use of bidentate allenyl imide and weakly activated and less steric hindered alkene pair by utilizing chiral magnesium(II) complex of N,N′-dioxide, which could provide through-space dispersion interactions to orientate the arrangement of the alkene. This protocol allows the generation of a series of axially chiral cyclobutenes and four-membered ring-containing spirocycles (80 examples) in high yield (up to 99 %) with excellent enantioselectivity (up to >99 % ee), and the late-stage modification of biologically active molecules as well. Experimental studies and DFT calculations revealed that this [2+2] cycloaddition proceeded via a stepwise mechanism involving a short-lived zwitterionic intermediate. The π-π interaction between the alkenes and the amide moiety in the ligand was crucial for the enantiocontrol.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202211596-sup-0001-C1.cif231.6 KB | Supporting Information |
| anie202211596-sup-0001-C54.cif234.3 KB | Supporting Information |
| anie202211596-sup-0001-E7.cif291.9 KB | Supporting Information |
| anie202211596-sup-0001-misc_information.pdf19.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews on cyclobutane motifs:
- 1aV. M. Dembitsky, Phytomedicine 2014, 21, 1559–1581;
- 1bM. A. Beniddir, L. Evanno, D. Joseph, A. Skiredj, E. Poupon, Nat. Prod. Rep. 2016, 33, 820–842;
- 1cJ. S. Li, K. Gao, M. Bian, H. F. Ding, Org. Chem. Front. 2020, 7, 136–154.
- 2
- 2aC. M. Marson, Chem. Soc. Rev. 2011, 40, 5514–5533;
- 2bM. R. Bauer, P. D. Fruscia, S. C. C. Lucas, I. N. Michaelides, J. E. Nelson, R. I. Storer, B. C. Whitehurst, RSC Med. Chem. 2021, 12, 448–471.
- 3For selected reviews:
- 3aE. Lee-Ruff, G. Mladenova, Chem. Rev. 2003, 103, 1449–1484;
- 3bJ. C. Namyslo, D. E. Kaufmann, Chem. Rev. 2003, 103, 1485–1538;
- 3cT. Seiser, T. Saget, D. N. Tran, N. Cramer, Angew. Chem. Int. Ed. 2011, 50, 7740–7752; Angew. Chem. 2011, 123, 7884–7896.
- 4For selected reviews on synthesis strategies of cyclobutanes:
- 4aA. Misale, S. Niyomchon, N. Maulide, Acc. Chem. Res. 2016, 49, 2444–2458;
- 4bM. Wang, P. Lu, Org. Chem. Front. 2018, 5, 254–259;
- 4cG. Otárola, J. J. Vaquero, E. Merino, M. A. Fernández-Rodríguez, Catalysts 2020, 10, 1178–1224.
- 5For selected reviews on enantioselective [2+2] cycloadditions:
- 5aF. Secci, A. Frongia, P. P. Piras, Molecules 2013, 18, 15541–15572;
- 5bY. Xu, M. L. Conner, M. K. Brown, Angew. Chem. Int. Ed. 2015, 54, 11918–11928; Angew. Chem. 2015, 127, 12086–12097;
- 5cK.-G. Wen, Y.-Y. Peng, X.-P. Zeng, Org. Chem. Front. 2020, 7, 2576–2597.
- 6For selected reviews on [2+2] cycloaddition of allenes:
- 6aB. Alcaide, P. Almendros, C. Aragoncillo, Chem. Soc. Rev. 2010, 39, 783–816;
- 6bM. R. Fructos, A. Prieto, Tetrahedron 2016, 72, 355–369;
- 6cJ. L. Mascareñas, I. Varela, F. López, Acc. Chem. Res. 2019, 52, 465–479;
- 6dM. Mato, A. Franchino, C. García-Morales, A. M. Echavarren, Chem. Rev. 2021, 121, 8613–8684.
- 7For selected examples on gold-catalyzed enantioselective [2+2] cycloaddition of allenes, see:
- 7aM. R. Luzung, P. Mauleón, F. D. Toste, J. Am. Chem. Soc. 2007, 129, 12402–12403;
- 7bH. Teller, S. Flügge, R. Goddard, A. Fürstner, Angew. Chem. Int. Ed. 2010, 49, 1949–1953; Angew. Chem. 2010, 122, 1993–1997;
- 7cA. Z. González, D. Benitez, E. Tkatchouk, W. A. Goddard, F. D. Toste, J. Am. Chem. Soc. 2011, 133, 5500–5507;
- 7dS. Suárez-Pantiga, C. Hernández-Díaz, E. Rubio, J. M. González, Angew. Chem. Int. Ed. 2012, 51, 11552–11555; Angew. Chem. 2012, 124, 11720–11723;
- 7eH. Teller, M. Corbet, L. Mantilli, G. Gopakumar, R. Goddard, W. Thiel, A. Fürstner, J. Am. Chem. Soc. 2012, 134, 15331–15342;
- 7fP. Mauleón, ChemCatChem 2013, 5, 2149–2151;
- 7gY. D. Wang, P. C. Zhang, Y. Liu, F. Xia, J. L. Zhang, Chem. Sci. 2015, 6, 5564–5570;
- 7hM. Q. Jia, M. Monari, Q.-Q. Yang, M. Bandini, Chem. Commun. 2015, 51, 2320–2323;
- 7iH. X. Hu, Y. D. Wang, D. Y. Qian, Z.-M. Zhang, L. Liu, J. L. Zhang, Org. Chem. Front. 2016, 3, 759–763;
- 7jW. Huang, Y.-C. Zhang, R. Jin, B.-L. Chen, Z. L. Chen, Organometallics 2018, 37, 3196–3209;
- 7kV. Magné, Y. Sanogo, C. S. Demmer, P. Retailleau, A. Marinetti, X. Guinchard, A. Voituriez, ACS Catal. 2020, 10, 8141–8148; For related non-enantioselective [2+2] cycloadditions, see:
- 7lH. Faustino, P. Bernal, L. Castedo, F. López, J. L. Mascareñas, Adv. Synth. Catal. 2012, 354, 1658–1664;
- 7mS. Suárez-Pantiga, C. Hernández-Díaz, M. Piedrafita, E. Rubio, J. M. González, Adv. Synth. Catal. 2012, 354, 1651–1657;
- 7nX.-X. Li, L.-L. Zhu, W. Zhou, Z. L. Chen, Org. Lett. 2012, 14, 436–439;
- 7oS. Montserrat, H. Faustino, A. Lledós, J. L. Mascareñas, F. López, G. Ujaque, Chem. Eur. J. 2013, 19, 15248–15260;
- 7pP. Bernal-Albert, H. Faustino, A. Gimeno, G. Asensio, J. L. Mascareñas, F. López, Org. Lett. 2014, 16, 6196–6199.
- 8For selected examples on enantioselective [2+2] cycloaddition of allenoates:
- 8aM. L. Conner, Y. Xu, M. K. Brown, J. Am. Chem. Soc. 2015, 137, 3482–3485;
- 8bJ. M. Wahl, M. L. Conner, M. K. Brown, J. Am. Chem. Soc. 2018, 140, 15943–15949;
- 8cJ. M. Wahl, M. L. Conner, M. K. Brown, Angew. Chem. Int. Ed. 2018, 57, 4647–4651; Angew. Chem. 2018, 130, 4737–4741;
- 8dE. N. Hancock, E. L. Kuker, D. J. Tantillo, M. K. Brown, Angew. Chem. Int. Ed. 2020, 59, 436–441; Angew. Chem. 2020, 132, 444–449;
- 8eR. Y. Guo, B. P. Witherspoon, M. K. Brown, J. Am. Chem. Soc. 2020, 142, 5002–5006.
- 9For reviews on chiral N,N′-dioxides:
- 9aX. H. Liu, L. L. Lin, X. M. Feng, Acc. Chem. Res. 2011, 44, 574–587;
- 9bX. H. Liu, L. L. Lin, X. M. Feng, Org. Chem. Front. 2014, 1, 298–302;
- 9cX. H. Liu, H. F. Zheng, Y. Xia, L. L. Lin, X. M. Feng, Acc. Chem. Res. 2017, 50, 2621–2631;
- 9dX. H. Liu, S. X. Dong, L. L. Lin, X. M. Feng, Chin. J. Chem. 2018, 36, 791–797;
- 9eZ. Wang, X. H. Liu, X. M. Feng, Aldrichimica Acta 2020, 53, 3–10;
- 9fM.-Y. Wang, W. Li, Chin. J. Chem. 2021, 39, 969–984; For recent examples:
- 9gX. B. Lin, Z. Tan, W. K. Yang, W. Yang, X. H. Liu, X. M. Feng, CCS Chem. 2021, 3, 1423–1433;
- 9hJ. Xu, Z. W. Zhong, M. Y. Jiang, Y. Q. Zhou, X. H. Liu, X. M. Feng, CCS Chem. 2021, 3, 1894–1902;
- 9iF. Tan, M. P. Pu, J. He, J. Z. Li, J. Yang, S. X. Dong, X. H. Liu, Y.-D. Wu, X. M. Feng, J. Am. Chem. Soc. 2021, 143, 2394–2402;
- 9jW. Yang, M. P. Pu, X. B. Lin, M. Chen, Y. J. Song, X. H. Liu, Y.-D. Wu, X. M. Feng, J. Am. Chem. Soc. 2021, 143, 9648–9656;
- 9kG. H. Pan, C. L. He, M. Chen, Q. Xiong, W. D. Cao, X. M. Feng, CCS Chem. 2022, 4, 2000–2008.
- 10For selected examples on Lewis acid catalyzed enantioselective [2+2] cycloadditions:
- 10aY. Hayashi, S. Niihata, K. Narasaka, Chem. Lett. 1990, 19, 2091–2094;
- 10bT. A. Engler, M. A. Letavic, J. P. Reddy, J. Am. Chem. Soc. 1991, 113, 5068–5070;
- 10cK. Narasaka, Y. Hayashi, H. Shimadzu, S. Niihata, J. Am. Chem. Soc. 1992, 114, 8869–8885;
- 10dK. Narasaka, K. Hayashi, Y. Hayashi, Tetrahedron 1994, 50, 4529–4542;
- 10eT. A. Engler, M. A. Letavic, R. Iyengar, K. O. LaTessa, J. P. Reddy, J. Org. Chem. 1999, 64, 2391–2405;
- 10fJ.-L. Hu, L.-W. Feng, L. J. Wang, Z. W. Xie, Y. Tang, X. G. Li, J. Am. Chem. Soc. 2016, 138, 13151–13154;
- 10gC. García-Morales, B. Ranieri, I. Escofet, L. López-Suarez, C. Obradors, A. I. Konovalov, A. M. Echavarren, J. Am. Chem. Soc. 2017, 139, 13628–13631;
- 10hL. Zeng, J. J. Xu, D. S. Zhang, Z. L. Yan, G. L. Cheng, W. D. Rao, L. Z. Gao, Angew. Chem. Int. Ed. 2020, 59, 21890–21894; Angew. Chem. 2020, 132, 22074–22078;
- 10iM. Zhang, X.-C. Wang, Angew. Chem. Int. Ed. 2021, 60, 17185–17190; Angew. Chem. 2021, 133, 17322–17327.
- 11For selected reviews on photochemical [2+2] cycloadditions:
- 11aJ. D. Winkler, C. M. Bowen, F. Liotta, Chem. Rev. 1995, 95, 2003–2020;
- 11bS. Poplata, A. Tröster, Y.-Q. Zou, T. Bach, Chem. Rev. 2016, 116, 9748–9815;
- 11cD. Sarkar, N. Bera, S. Ghosh, Eur. J. Org. Chem. 2020, 1310–1326; For selected examples:
- 11dR. Brimioulle, T. Bach, Science 2013, 342, 840–843;
- 11eT. R. Blum, Z. D. Miller, D. M. Bates, I. A. Guzei, T. P. Yoon, Science 2016, 354, 1391–1395;
- 11fX. Q. Huang, T. R. Quinn, K. Harms, R. D. Webster, L. L. Zhang, O. Wiest, E. Meggers, J. Am. Chem. Soc. 2017, 139, 9120–9123;
- 11gZ. D. Miller, B. J. Lee, T. P. Yoon, Angew. Chem. Int. Ed. 2017, 56, 11891–11895; Angew. Chem. 2017, 129, 12053–12057;
- 11hN. F. Hu, H. Jung, Y. Zheng, J. Lee, L. L. Zhang, Z. Ullah, X. L. Xie, K. Harms, M.-H. Baik, E. Meggers, Angew. Chem. Int. Ed. 2018, 57, 6242–6246; Angew. Chem. 2018, 130, 6350–6354;
- 11iS. Poplata, T. Bach, J. Am. Chem. Soc. 2018, 140, 3228–3231;
- 11jM. E. Daub, H. Jung, B. J. Lee, J. Won, M.-H. Baik, T. P. Yoon, J. Am. Chem. Soc. 2019, 141, 9543–9547;
- 11kS. Cao, Z. Q. Ye, Y. H. Chen, Y.-M. Lin, J. H. Fang, Y. J. Wang, B. X. Yang, L. Gong, CCS Chem. 2022, 4, 3122–3133.
- 12For selected examples of our previous work about [2+2] cycloadditions :
- 12aT. F. Kang, S. L. Ge, L. L. Lin, Y. Lu, X. H. Liu, X. M. Feng, Angew. Chem. Int. Ed. 2016, 55, 5541–5544; Angew. Chem. 2016, 128, 5631–5634;
- 12bH. F. Zheng, C. R. Xu, Y. Wang, T. F. Kang, X. H. Liu, L. L. Lin, X. M. Feng, Chem. Commun. 2017, 53, 6585–6588;
- 12cH. Yu, S. X. Dong, Q. Yao, L. Chen, D. Zhang, X. H. Liu, X. M. Feng, Chem. Eur. J. 2018, 24, 19361–19367;
- 12dX. Zhong, Q. Tang, P. F. Zhou, Z. W. Zhong, S. X. Dong, X. H. Liu, X. M. Feng, Chem. Commun. 2018, 54, 10511–10514;
- 12eX. Zhong, J. Q. Tan, J. L. Qiao, Y. Q. Zhou, C. D. Lv, Z. S. Su, S. X. Dong, X. M. Feng, Chem. Sci. 2021, 12, 9991–9997.
- 13For selected reviews and books on axially chiral cyclobutenes:
- 13aT. Rein, T. M. Pedersen, Synthesis 2002, 579–594;
- 13bB. Skrobo, J. D. Rolfes, J. Deska, Tetrahedron 2016, 72, 1257–1275;
- 13cJ. K. Cheng, S.-H. Xiang, S. Y. Li, L. Ye, B. Tan, Chem. Rev. 2021, 121, 4805–4902;
- 13dB. Tan, Axially Chiral Compounds: Asymmetric Synthesis and Applications, Wiley-VCH, Weinheim, 2021. For selected examples:
10.1002/9783527825172 Google Scholar
- 13eR. Agudo, G.-D. Roiban, M. T. Reetz, J. Am. Chem. Soc. 2013, 135, 1665–1668;
- 13fS. Crotti, N. D. Iorio, C. Artusi, A. Mazzanti, P. Righi, G. Bencivenni, Org. Lett. 2019, 21, 3013–3017;
- 13gS. K. Nimmagadda, S. C. Mallojjala, L. Woztas, S. E. Wheeler, J. C. Antilla, Angew. Chem. Int. Ed. 2017, 56, 2454–2458; Angew. Chem. 2017, 129, 2494–2498.
- 14For selected reviews on spirocyclic cyclobutanes:
- 14aE. M. Carreira, T. C. Fessard, Chem. Rev. 2014, 114, 8257–8322;
- 14bY. J. Zheng, C. M. Tice, S. B. Singh, Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682;
- 14cP.-W. Xu, J.-S. Yu, C. Chen, Z.-Y. Cao, F. Zhou, J. Zhou, ACS Catal. 2019, 9, 1820–1882.
- 15Deposition numbers 2077428 (C1) and 2181395 (E7) contains the supplentary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 16
- 16aA. Ehrlich, S. Uhl, K. Ioannidis, M. Hofree, B. R. tenOever, Y. Nahmias, Cell Metab. 2020, https://doi.org/10.2139/ssrn.3650499;
- 16bS. P. Davies, C. J. Mycroft-West, I. Pagani, H. J. Hill, Y.-H. Chen, R. Karlsson, I. Bagdonaite, S. E. Guimond, Z. Stamataki, M. A. De Lima, J. E. Turnbull, Z. Yang, E. Vicenzi, M. A. Skidmore, F. L. Khanim, A. Richardson, Front. Pharmacol. 2021, https://doi.org/10.3389/fphar.2021.660490.
- 17For selected reviews and book:
- 17aR. Hoffmann, R. B. Woodward, Acc. Chem. Res. 1968, 1, 17–22;
- 17bR. B. Woodward, R. Hoffmann, Angew. Chem. Int. Ed. Engl. 1969, 8, 781–853; Angew. Chem. 1969, 81, 797–869;
- 17cI. Fleming, Molecular Orbitals and Organic Chemical Reactions, Wiley, Chichester, 2009;
10.1002/9780470684306 Google Scholar
- 17dR. Gompper, Angew. Chem. Int. Ed. Engl. 1969, 8, 312–327; Angew. Chem. 1969, 81, 348–363. For selected example:
- 17eX. B. Wang, K. N. Houk, J. Am. Chem. Soc. 1990, 112, 1754–1756.
- 18The difference between Z- and E- diastereomers was H and D, which could not be separated by HPLC or UPC2 analysis. Thus, the ee values in Scheme 3 correspond to the mixture of Z/E diastereomers.