Multistate Circularly Polarized Luminescence Switching through Stimuli-Induced Co-Conformation Regulations of Pyrene-Functionalized Topologically Chiral [2]Catenane
Yu Wang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes &, Shanghai Frontiers Science Center of Molecule Intelligent Syntheses &, Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorJiacheng Gong
Shanghai Key Laboratory of Green Chemistry and Chemical Processes &, Shanghai Frontiers Science Center of Molecule Intelligent Syntheses &, Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorDr. Xianwei Wang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes &, Shanghai Frontiers Science Center of Molecule Intelligent Syntheses &, Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorDr. Wei-Jian Li
Shanghai Key Laboratory of Green Chemistry and Chemical Processes &, Shanghai Frontiers Science Center of Molecule Intelligent Syntheses &, Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorDr. Xu-Qing Wang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes &, Shanghai Frontiers Science Center of Molecule Intelligent Syntheses &, Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiao He
Shanghai Key Laboratory of Green Chemistry and Chemical Processes &, Shanghai Frontiers Science Center of Molecule Intelligent Syntheses &, Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Wei Wang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes &, Shanghai Frontiers Science Center of Molecule Intelligent Syntheses &, Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hai-Bo Yang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes &, Shanghai Frontiers Science Center of Molecule Intelligent Syntheses &, Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Institute of Eco-Chongming, Shanghai, 202162 China
Search for more papers by this authorYu Wang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes &, Shanghai Frontiers Science Center of Molecule Intelligent Syntheses &, Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorJiacheng Gong
Shanghai Key Laboratory of Green Chemistry and Chemical Processes &, Shanghai Frontiers Science Center of Molecule Intelligent Syntheses &, Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorDr. Xianwei Wang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes &, Shanghai Frontiers Science Center of Molecule Intelligent Syntheses &, Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorDr. Wei-Jian Li
Shanghai Key Laboratory of Green Chemistry and Chemical Processes &, Shanghai Frontiers Science Center of Molecule Intelligent Syntheses &, Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorDr. Xu-Qing Wang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes &, Shanghai Frontiers Science Center of Molecule Intelligent Syntheses &, Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiao He
Shanghai Key Laboratory of Green Chemistry and Chemical Processes &, Shanghai Frontiers Science Center of Molecule Intelligent Syntheses &, Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Wei Wang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes &, Shanghai Frontiers Science Center of Molecule Intelligent Syntheses &, Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hai-Bo Yang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes &, Shanghai Frontiers Science Center of Molecule Intelligent Syntheses &, Chang-Kung Chuang Institute, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 China
Institute of Eco-Chongming, Shanghai, 202162 China
Search for more papers by this authorGraphical Abstract
Abstract
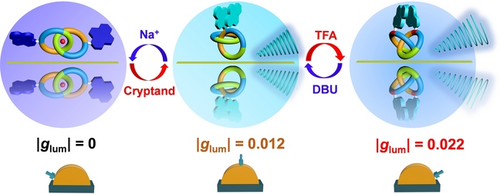
Aiming at the construction of novel circularly polarized luminescence (CPL) switches with multiple switchable emission states and high dissymmetry factors (glum), topologically chiral [2]catenanes were employed as the key platform to construct a novel multistate CPL switching system. Taking advantage of the precise co-conformation regulations of the resultant pyrene-functionalized [2]catenanes under different external stimuli, reversible transformations between three emission states with different CPL performances, i.e. the initial “closed” form with a |glum| value of 0.012, the “open” form with an almost complete turn-off of CPL emission, and the “protonated” form with a boosted |glum| value of 0.022, were successfully realized. This study demonstrates the successful construction of not only the first topological chirality-based CPL switch, but also a novel bidirectional CPL switch, thus providing a promising platform for the construction of novel chiral materials.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202210542-sup-0001-misc_information.pdf3.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. Longhi, E. Castiglioni, J. Koshoubu, G. Mazzeo, S. Abbate, Chirality 2016, 28, 696–707;
- 1bD.-W. Zhang, M. Li, C.-F. Chen, Chem. Soc. Rev. 2020, 49, 1331–1343;
- 1cL. E. MacKenzie, R. Pal, Nat. Chem. Rev. 2021, 5, 109–124;
- 1dY. Sang, J. Han, T. Zhao, P. Duan, M. Liu, Adv. Mater. 2020, 32, 1900110;
- 1eZ.-L. Gong, X. Zhu, Z. Zhou, S.-W. Zhang, D. Yang, B. Zhao, Y.-P. Zhang, J. Deng, Y. Cheng, Y.-X. Zheng, S.-Q. Zang, H. Kuang, P. Duan, M. Yuan, C.-F. Chen, Y. S. Zhao, Y.-W. Zhong, B. Z. Tang, M. Liu, Sci. China Chem. 2021, 64, 2060–2104.
- 2
- 2aR. Carr, N. H. Evans, D. Parker, Chem. Soc. Rev. 2012, 41, 7673–7686;
- 2bF. Zinna, L. Di Bari, Chirality 2015, 27, 1–13;
- 2cY. Zhou, H. Li, T. Zhu, T. Gao, P. Yan, J. Am. Chem. Soc. 2019, 141, 19634–19643;
- 2dS.-J. Hu, X.-Q. Guo, L.-P. Zhou, D.-N. Yan, P.-M. Cheng, L.-X. Cai, X.-Z. Li, Q.-F. Sun, J. Am. Chem. Soc. 2022, 144, 4244–4253.
- 3
- 3aE. M. Sánchez-Carnerero, A. R. Agarrabeitia, F. Moreno, B. L. Maroto, G. Muller, M. J. Ortiz, S. de la Moya, Chem. Eur. J. 2015, 21, 13488–13500;
- 3bF. Pop, N. Zigon, N. Avarvari, Chem. Rev. 2019, 119, 8435–8478;
- 3cS. Tong, J.-T. Li, D.-D. Liang, Y.-E. Zhang, Q.-Y. Feng, X. Zhang, J. Zhu, M.-X. Wang, J. Am. Chem. Soc. 2020, 142, 14432–14436;
- 3dJ.-M. Teng, D.-W. Zhang, C.-F. Chen, ChemPhotoChem 2022, 6, e202100228.
- 4
- 4aW. Ma, L. Xu, A. F. de Moura, X. Wu, H. Kuang, C. Xu, N. A. Kotov, Chem. Rev. 2017, 117, 8041–8093;
- 4bJ.-X. Gao, W.-Y. Zhang, Z.-G. Wu, Y.-X. Zheng, D.-W. Fu, J. Am. Chem. Soc. 2020, 142, 4756–4761;
- 4cM. Deng, N. F. M. Mukthar, N. D. Schley, G. Ung, Angew. Chem. Int. Ed. 2020, 59, 1228–1231; Angew. Chem. 2020, 132, 1244–1247;
- 4dM.-M. Zhang, X.-Y. Dong, Z.-Y. Wang, X.-M. Luo, J.-H. Huang, S.-Q. Zang, T. C. W. Mak, J. Am. Chem. Soc. 2021, 143, 6048–6053;
- 4eH. Wu, X. He, B. Yang, C.-C. Li, L. Zhao, Angew. Chem. Int. Ed. 2021, 60, 1535–1539; Angew. Chem. 2021, 133, 1559–1563;
- 4fM.-M. Zhang, X.-Y. Dong, Z.-Y. Wang, H.-Y. Li, S.-J. Li, X. Zhao, S.-Q. Zang, Angew. Chem. Int. Ed. 2020, 59, 10052–10058; Angew. Chem. 2020, 132, 10138–10144;
- 4gC. Zhang, S. Li, X.-Y. Dong, S.-Q. Zang, Aggregate 2021, 2, e48.
- 5
- 5aE. Peeters, M. P. T. Christiaans, R. A. J. Janssen, H. F. M. Schoo, H. P. J. M. Dekkers, E. W. Meijer, J. Am. Chem. Soc. 1997, 119, 9909–9910;
- 5bA. Satrijo, S. C. J. Meskers, T. M. Swager, J. Am. Chem. Soc. 2006, 128, 9030–9031;
- 5cL. Wang, L. Yin, W. Zhang, X. Zhu, M. Fujiki, J. Am. Chem. Soc. 2017, 139, 13218–13226;
- 5dO. Oki, C. Kulkarni, H. Yamagishi, S. C. J. Meskers, Z. Lin, J. Huang, E. W. Meijer, Y. Yamamoto, J. Am. Chem. Soc. 2021, 143, 8772–8779;
- 5eL. Hu, X. Zhu, C. Yang, M. Liu, Angew. Chem. Int. Ed. 2022, 61, e202114759; Angew. Chem. 2022, 134, e202114759;
- 5fH. Fan, K. Li, T. Lu, X. Zhu, L. Zhang, M. Liu, Angew. Chem. Int. Ed. 2022, 61, e202200727; Angew. Chem. 2022, 134, e202200727.
- 6
- 6aL. Zhang, H.-X. Wang, S. Li, M. Liu, Chem. Soc. Rev. 2020, 49, 9095–9120;
- 6bJ.-L. Ma, Q. Peng, C.-H. Zhao, Chem. Eur. J. 2019, 25, 15441–15454;
- 6cY. Gao, C. Ren, X. Lin, T. He, Front. Chem. 2020, 8, 458.
- 7
- 7aA. Homberg, E. Brun, F. Zinna, S. Pascal, M. Górecki, L. Monnier, C. Besnard, G. Pescitelli, L. Di Bari, J. Lacour, Chem. Sci. 2018, 9, 7043–7052;
- 7bK. Takaishi, M. Yasui, T. Ema, J. Am. Chem. Soc. 2018, 140, 5334–5338;
- 7cA. H. G. David, R. Casares, J. M. Cuerva, A. G. Campana, V. Blanco, J. Am. Chem. Soc. 2019, 141, 18064–18074;
- 7dS. Wang, D. Hu, X. Guan, S. Cai, G. Shi, Z. Shuai, J. Zhang, Q. Peng, X. Wan, Angew. Chem. Int. Ed. 2021, 60, 21918–21926; Angew. Chem. 2021, 133, 22089–22097;
- 7eS. Fa, T. Tomita, K. Wada, K. Yasuhara, S. Ohtani, K. Kato, M. Gon, K. Tanaka, T. Kakuta, T.-a. Yamagishi, T. Ogoshi, Chem. Sci. 2022, 13, 5846–5853.
- 8W.-J. Li, Q. Gu, X.-Q. Wang, D.-Y. Zhang, Y.-T. Wang, X. He, W. Wang, H.-B. Yang, Angew. Chem. Int. Ed. 2021, 60, 9507–9515; Angew. Chem. 2021, 133, 9593–9601.
- 9
- 9aC. J. Bruns, J. F. Stoddart, The Nature of the Mechanical Bond: From Molecules to Machines, Wiley, Hoboken, 2016;
10.1002/9781119044123 Google Scholar
- 9bD. Sluysmans, J. F. Stoddart, Trends Chem. 2019, 1, 185–197;
- 9cJ. F. Stoddart, Angew. Chem. Int. Ed. 2017, 56, 11094–11125; Angew. Chem. 2017, 129, 11244–11277;
- 9dA. Goujon, E. Moulin, G. Fuks, N. Giuseppone, CCS Chem. 2019, 1, 83–96;
- 9eA. W. Heard, S. M. Goldup, ACS Cent. Sci. 2020, 6, 117–128;
- 9fJ. R. J. Maynard, S. M. Goldup, Chem 2020, 6, 1914–1932;
- 9gA. W. Heard, J. M. Suárez, S. M. Goldup, Nat. Chem. Rev. 2022, 6, 182–196;
- 9hA. H. G. David, J. F. Stoddart, Isr. J. Chem. 2021, 61, 608–621;
- 9iA. Inthasot, S.-T. Tung, S.-H. Chiu, Acc. Chem. Res. 2018, 51, 1324–1337;
- 9jW. Wang, L.-J. Chen, X.-Q. Wang, B. Sun, X. Li, Y. Zhang, J. Shi, Y. Yu, L. Zhang, M. Liu, H.-B. Yang, Proc. Natl. Acad. Sci. USA 2015, 112, 5597–5601;
- 9kX.-Q. Wang, W.-J. Li, W. Wang, H.-B. Yang, Chem. Commun. 2018, 54, 13303–13318;
- 9lX.-Q. Wang, W. Wang, W.-J. Li, L.-J. Chen, R. Yao, G.-Q. Yin, Y.-X. Wang, Y. Zhang, J. Huang, H. Tan, Y. Yu, X. Li, L. Xu, H.-B. Yang, Nat. Commun. 2018, 9, 3190;
- 9mW. Wang, H.-B. Yang, Sci. Bull. 2020, 65, 1964–1965;
- 9nX.-Q. Wang, W.-J. Li, W. Wang, H.-B. Yang, Acc. Chem. Res. 2021, 54, 4091–4106;
- 9oX.-Q. Xu, X.-Q. Wang, W. Wang, Chin. Chem. Lett. 2022, https://doi.org/10.1016/j.cclet.2022.07.008.
- 10
- 10aK. Caprice, D. Pal, C. Besnard, B. Galmes, A. Frontera, F. B. L. Cougnon, J. Am. Chem. Soc. 2021, 143, 11957–11962;
- 10bS. Amano, M. Esposito, E. Kreidt, D. A. Leigh, E. Penocchio, B. M. W. Roberts, Nat. Chem. 2022, 14, 530–537;
- 10cS. Erbas-Cakmak, S. D. P. Fielden, U. Karaca, D. A. Leigh, C. T. McTernan, D. J. Tetlow, M. R. Wilson, Science 2017, 358, 340–343;
- 10dJ. E. M. Lewis, F. Modicom, S. M. Goldup, J. Am. Chem. Soc. 2018, 140, 4787–4791;
- 10eG. Gil-Ramírez, D. A. Leigh, A. J. Stephens, Angew. Chem. Int. Ed. 2015, 54, 6110–6150; Angew. Chem. 2015, 127, 6208–6249;
- 10fD. P. August, J. Jaramillo-Garcia, D. A. Leigh, A. Valero, I. J. Vitorica-Yrezabal, J. Am. Chem. Soc. 2021, 143, 1154–1161;
- 10gY. Deng, S. K.-M. Lai, L. Kong, H. Y. Au-Yeung, Chem. Commun. 2021, 57, 2931–2934;
- 10hY. Wang, S. Lu, X.-Q. Wang, Y.-F. Niu, H. Wang, W. Wang, Org. Chem. Front. 2021, 8, 4994–5001.
- 11
- 11aT.-Y. Tai, Y.-H. Liu, C.-C. Lai, S.-M. Peng, S.-H. Chiu, Org. Lett. 2019, 21, 5708–5712;
- 11bD. K. Mitchell, J. P. Sauvage, Angew. Chem. Int. Ed. Engl. 1988, 27, 930–931; Angew. Chem. 1988, 100, 985–987;
- 11cJ. C. Chambron, D. K. Mitchell, J. P. Sauvage, J. Am. Chem. Soc. 1992, 114, 4625–4631;
- 11dC. Yamamoto, Y. Okamoto, T. Schmidt, R. Jäger, F. Vögtle, J. Am. Chem. Soc. 1997, 119, 10547–10548;
- 11eC. P. McArdle, S. Van, M. C. Jennings, R. J. Puddephatt, J. Am. Chem. Soc. 2002, 124, 3959–3965;
- 11fM. Denis, J. E. M. Lewis, F. Modicom, S. M. Goldup, Chem 2019, 5, 1512–1520;
- 11gE. M. G. Jamieson, F. Modicom, S. M. Goldup, Chem. Soc. Rev. 2018, 47, 5266–5311.
- 12
- 12aJ.-P. Sauvage, Angew. Chem. Int. Ed. 2017, 56, 11080–11093; Angew. Chem. 2017, 129, 11228–11242;
- 12bE. Flapan, When topology meets chemistry: a topological look at molecular chirality, Cambridge University Press, Cambridge, 2000;
10.1017/CBO9780511626272 Google Scholar
- 12cD. Buck, E. Flapan, Applications of knot theory, American Mathematical Society, Providence, 2009;
10.1090/psapm/066 Google Scholar
- 12dJ. C. Chambron, C. Dietrich-Buchecker, J. P. Sauvage, Top. Curr. Chem. 1993, 165, 131–162.
- 13
- 13aM. Inouye, K. Hayashi, Y. Yonenaga, T. Itou, K. Fujimoto, T.-a. Uchida, M. Iwamura, K. Nozaki, Angew. Chem. Int. Ed. 2014, 53, 14392–14396; Angew. Chem. 2014, 126, 14620–14624;
- 13bM. Inouye, A. Yoshizawa, M. Shibata, Y. Yonenaga, K. Fujimoto, T. Sakata, S. Matsumoto, M. Shiro, Org. Lett. 2016, 18, 1960–1963;
- 13cK. Hayashi, Y. Miyaoka, Y. Ohishi, T.-a. Uchida, M. Iwamura, K. Nozaki, M. Inouye, Chem. Eur. J. 2018, 24, 14613–14616.
- 14
- 14aK. Takaishi, R. Takehana, T. Ema, Chem. Commun. 2018, 54, 1449–1452;
- 14bK. Takaishi, K. Iwachido, R. Takehana, M. Uchiyama, T. Ema, J. Am. Chem. Soc. 2019, 141, 6185–6190;
- 14cK. Takaishi, K. Iwachido, T. Ema, J. Am. Chem. Soc. 2020, 142, 1774–1779;
- 14dK. Takaishi, S. Murakami, K. Iwachido, T. Ema, Chem. Sci. 2021, 12, 14570–14576;
- 14eK. Takaishi, S. Murakami, F. Yoshinami, T. Ema, Angew. Chem. Int. Ed. 2022, 61, e202204609; Angew. Chem. 2022, 134, e202204609.
- 15
- 15aD. Niu, Y. Jiang, L. Ji, G. Ouyang, M. Liu, Angew. Chem. Int. Ed. 2019, 58, 5946–5950; Angew. Chem. 2019, 131, 6007–6011;
- 15bJ. Zhu, S. Chen, C. He, J. Am. Chem. Soc. 2021, 143, 5301–5307;
- 15cS. Hu, L. Hu, X. Zhu, Y. Wang, M. Liu, Angew. Chem. Int. Ed. 2021, 60, 19451–19457; Angew. Chem. 2021, 133, 19600–19606;
- 15dH. Shigemitsu, K. Kawakami, Y. Nagata, R. Kajiwara, S. Yamada, T. Mori, T. Kida, Angew. Chem. Int. Ed. 2022, 61, e202114700; Angew. Chem. 2022, 134, e202114700.
- 16T. Mori, Circularly Polarized Luminescence of Isolated Small Organic Molecules, Springer Nature Singapore Pte Ltd., Gateway East, Singapore, 2020.
10.1007/978-981-15-2309-0 Google Scholar
- 17
- 17aY. Ohishi, M. Inouye, Tetrahedron Lett. 2019, 60, 151232;
- 17bK. Kano, H. Matsumoto, S. Hashimoto, M. Sisido, Y. Imanishi, J. Am. Chem. Soc. 1985, 107, 6117–6118;
- 17cT. Nishikawa, N. Tajima, M. Kitamatsu, M. Fujiki, Y. Imai, Org. Biomol. Chem. 2015, 13, 11426–11431;
- 17dY. Hashimoto, T. Nakashima, D. Shimizu, T. Kawai, Chem. Commun. 2016, 52, 5171–5174;
- 17eC. Tu, W. Wu, W. Liang, D. Zhang, W. Xu, S. Wan, W. Lu, C. Yang, Angew. Chem. Int. Ed. 2022, 61, e202203541; Angew. Chem. 2022, 134, e202203541;
- 17fM. Kazem-Rostami, A. Orte, A. M. Ortuño, A. H. G. David, I. Roy, D. Miguel, A. Garci, C. M. Cruz, C. L. Stern, J. M. Cuerva, J. F. Stoddart, J. Am. Chem. Soc. 2022, 144, 9380–9389.
- 18
- 18aA. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher, T. E. Rice, Chem. Rev. 1997, 97, 1515–1566;
- 18bA. W. Czarnik, Acc. Chem. Res. 1994, 27, 302–308;
- 18cF. Galindo, M. I. Burguete, L. Vigara, S. V. Luis, N. Kabir, J. Gavrilovic, D. A. Russell, Angew. Chem. Int. Ed. 2005, 44, 6504–6508; Angew. Chem. 2005, 117, 6662–6666;
- 18dH. M. Kim, M. J. An, J. H. Hong, B. H. Jeong, O. Kwon, J.-Y. Hyon, S.-C. Hong, K. J. Lee, B. R. Cho, Angew. Chem. Int. Ed. 2008, 47, 2231–2234; Angew. Chem. 2008, 120, 2263–2266;
- 18eT. Myochin, K. Kiyose, K. Hanaoka, H. Kojima, T. Terai, T. Nagano, J. Am. Chem. Soc. 2011, 133, 3401–3409;
- 18fS. Takahashi, Y. Kagami, K. Hanaoka, T. Terai, T. Komatsu, T. Ueno, M. Uchiyama, I. Koyama-Honda, N. Mizushima, T. Taguchi, H. Arai, T. Nagano, Y. Urano, J. Am. Chem. Soc. 2018, 140, 5925–5933.