Regioselective Transition-Metal-Free C(sp2)−H Borylation: A Subject of Practical and Ongoing Interest in Synthetic Organic Chemistry
Corresponding Author
Dr. Supriya Rej
Department of Applied Chemistry, Faculty of Engineering and Research Center for Environmental Preservation, Osaka University, Suita, Osaka, 565-0871 Japan
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Contribution: Writing - original draft (lead)
Search for more papers by this authorCorresponding Author
Prof. Dr. Naoto Chatani
Department of Applied Chemistry, Faculty of Engineering and Research Center for Environmental Preservation, Osaka University, Suita, Osaka, 565-0871 Japan
Contribution: Writing - review & editing (equal)
Search for more papers by this authorCorresponding Author
Dr. Supriya Rej
Department of Applied Chemistry, Faculty of Engineering and Research Center for Environmental Preservation, Osaka University, Suita, Osaka, 565-0871 Japan
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Contribution: Writing - original draft (lead)
Search for more papers by this authorCorresponding Author
Prof. Dr. Naoto Chatani
Department of Applied Chemistry, Faculty of Engineering and Research Center for Environmental Preservation, Osaka University, Suita, Osaka, 565-0871 Japan
Contribution: Writing - review & editing (equal)
Search for more papers by this authorGraphical Abstract
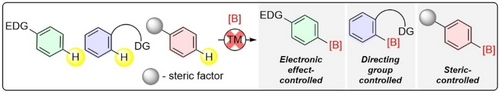
Recent developments in strategies for transition-metal-free regioselective C−H borylation are summarized in this Review. Strategies developed to control the regioselectivity of the electrophilic borylation, such as approaches controlled by electronic effects, auxiliaries, and steric factors, are also discussed. EDG=electron donating group, DG=directing group, TM=transition metal.
Abstract
Considerable advances have been made in the area of C−H functionalization in the last few decades. A number of approaches including both directed and nondirected strategies have been developed thus far. Among the various C−H functionalizations, C−H borylation is of special interest due to the wide applications of organoboron compounds. In this regard, various transition-metal-catalyzed regioselective strategies have been developed. However, the major concern regarding metal-catalyzed C−H borylation procedures is the requirement of a precious metal as well as the contamination by metal precursors in the desired products, which limit the application of this process in large-scale synthesis. Therefore, recent trends have involved the use of transition-metal-free systems. We summarize recent developments in transition-metal-free regioselective C−H borylation. We believe that this Review will help to increase interest in this field and stimulate further progress.
Conflict of interest
The authors declare no conflict of interest.
References
- 1M. H. Emmert, C. J. Legacy, Arene Chemistry: Reaction Mechanisms and Methods for Aromatic Compounds, Wiley, Hoboken, 2015, pp. 647–673.
- 2T. Gensch, M. N. Hopkinson, F. Glorius, J. Wencel-Delord, Chem. Soc. Rev. 2016, 45, 2900–2936.
- 3C. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes, M. Schnürch, Chem. Soc. Rev. 2018, 47, 6603–6743.
- 4S. Rej, Y. Ano, N. Chatani, Chem. Rev. 2020, 120, 1788–1887.
- 5U. Dutta, S. Maiti, T. Bhattacharya, D. Maiti, Science 2021, 372, eabd5992.
- 6S. Rej, A. Das, N. Chatani, Coord. Chem. Rev. 2021, 431, 213683.
- 7A. Ros, R. Fernández, J. M. Lassaletta, Chem. Soc. Rev. 2014, 43, 3229–3243.
- 8L. Veth, H. A. Grab, P. Dydio, Synthesis 2021, 54, A–Q.
- 9B. Su, J. F. Hartwig, Angew. Chem. Int. Ed. 2022, 61, e202113343; Angew. Chem. 2022, 134, e202113343.
- 10A. Suzuki, Angew. Chem. Int. Ed. 2011, 50, 6722–6737; Angew. Chem. 2011, 123, 6854–6869.
- 11I. P. Beletskaya, F. Alonso, V. Tyurin, Coord. Chem. Rev. 2019, 385, 137–173.
- 12D. W. Stephan, Org. Biomol. Chem. 2008, 6, 1535–1539.
- 13J. L. Carden, A. Dasgupta, R. L. Melen, Chem. Soc. Rev. 2020, 49, 1706–1725.
- 14V. Nori, F. Pesciaioli, A. Sinibaldi, G. Giorgianni, A. Carlone, Catalysts 2021, 12, 5.
- 15Z. M. Hudson, S. Wang, Acc. Chem. Res. 2009, 42, 1584–1596.
- 16F. Jäkle, Chem. Rev. 2010, 110, 3985–4022.
- 17C. R. Wade, A. E. J. Broomsgrove, S. Aldridge, F. P. Gabbaï, Chem. Rev. 2010, 110, 3958–3984.
- 18D. Li, H. Zhang, Y. Wang, Chem. Soc. Rev. 2013, 42, 8416–8433.
- 19H. C. Brown, T. E. Cole, Organometallics 1983, 2, 1316–1319.
- 20H. C. Brown, M. Srebnik, T. E. Cole, Organometallics 1986, 5, 2300–2303.
- 21O. Baron, P. Knochel, Angew. Chem. Int. Ed. 2005, 44, 3133–3135; Angew. Chem. 2005, 117, 3193–3195.
- 22C. Pintaric, S. Olivero, Y. Gimbert, P. Y. Chavant, E. Duñach, J. Am. Chem. Soc. 2010, 132, 11825–11827.
- 23W. K. Chow, O. Y. Yuen, P. Y. Choy, C. M. So, C. P. Lau, W. T. Wong, F. Y. Kwong, RSC Adv. 2013, 3, 12518–12539.
- 24A. J. J. Lennox, G. C. Lloyd-Jones, Chem. Soc. Rev. 2014, 43, 412–443.
- 25K. Kubota, H. Iwamoto, H. Ito, Org. Biomol. Chem. 2017, 15, 285–300.
- 26Y.-M. Tian, X.-N. Guo, H. Braunschweig, U. Radius, T. B. Marder, Chem. Rev. 2021, 121, 3561–3597.
- 27F.-G. Fontaine, É. Rochette, Acc. Chem. Res. 2018, 51, 454–464.
- 28Y. Li, X.-F. Wu, Angew. Chem. Int. Ed. 2020, 59, 1770–1774; Angew. Chem. 2020, 132, 1786–1790.
- 29S. A. Iqbal, J. Pahl, K. Yuan, M. J. Ingleson, Chem. Soc. Rev. 2020, 49, 4564–4591.
- 30T. S. De Vries, A. Prokofjevs, E. Vedejs, Chem. Rev. 2012, 112, 4246–4282.
- 31S. Bähr, M. Oestreich, Pure Appl. Chem. 2018, 90, 723–731.
- 32R. M. Roberts, Y. W. Han, C. H. Schmid, D. A. Davis, J. Am. Chem. Soc. 1959, 81, 640–643.
- 33E. L. Muetterties, J. Am. Chem. Soc. 1960, 82, 4163–4166.
- 34D. T. Hurd, J. Am. Chem. Soc. 1948, 70, 2053–2055.
- 35Z. J. Bujwid, W. Gerrard, M. F. Lappert, Chem. Ind. 1959, 1091.
- 36E. L. Muetterties, J. Am. Chem. Soc. 1959, 81, 2597.
- 37K. Niedenzu, J. W. Dawson, Angew. Chem. 1959, 71, 651.
- 38A. Del Grosso, R. G. Pritchard, C. A. Muryn, M. J. Ingleson, Organometallics 2010, 29, 241–249.
- 39A. Prokofjevs, J. W. Kampf, E. Vedejs, Angew. Chem. Int. Ed. 2011, 50, 2098–2101; Angew. Chem. 2011, 123, 2146–2149.
- 40A. Del Grosso, P. J. Singleton, C. A. Muryn, M. J. Ingleson, Angew. Chem. Int. Ed. 2011, 50, 2102–2106; Angew. Chem. 2011, 123, 2150–2154.
- 41A. Del Grosso, M. D. Helm, S. A. Solomon, D. Caras-Quintero, M. J. Ingleson, Chem. Commun. 2011, 47, 12459–12461.
- 42V. Bagutski, A. Del Grosso, J. A. Carrillo, I. A. Cade, M. D. Helm, J. R. Lawson, P. J. Singleton, S. A. Solomon, T. Marcelli, M. J. Ingleson, J. Am. Chem. Soc. 2013, 135, 474–487.
- 43S. A. Solomon, A. Del Grosso, E. R. Clark, V. Bagutski, J. J. W. McDouall, M. J. Ingleson, Organometallics 2012, 31, 1908–1916.
- 44A. Del Grosso, J. A. Carrillo, M. J. Ingleson, Chem. Commun. 2015, 51, 2878–2881.
- 45D. Wang, H. Minami, C. Wang, M. Uchiyama, Chem. Lett. 2015, 44, 1380–1382.
- 46S. Tanaka, Y. Saito, T. Yamamoto, T. Hattori, Org. Lett. 2018, 20, 1828–1831.
- 47V. Iashin, D. Berta, K. Chernichenko, M. Nieger, K. Moslova, I. Papai, T. Repo, Chem. Eur. J. 2020, 26, 13873–13879.
- 48Q. Yin, H. F. T. Klare, M. Oestreich, Angew. Chem. Int. Ed. 2017, 56, 3712–3717; Angew. Chem. 2017, 129, 3766–3771.
- 49F. Kitani, R. Takita, T. Imahori, M. Uchiyama, Heterocycles 2017, 95, 158–166.
- 50Y.-L. Liu, G. Kehr, C. G. Daniliuc, G. Erker, Chem. Eur. J. 2017, 23, 12141–12144.
- 51S. Zhang, Y. Han, J. He, Y. Zhang, J. Org. Chem. 2018, 83, 1377–1386.
- 52M.-A. Légaré, M.-A. Courtemanche, É. Rochette, F.-G. Fontaine, Science 2015, 349, 513–516.
- 53S. K. Bose, T. B. Marder, Science 2015, 349, 473–474.
- 54J. Légaré Lavergne, A. Jayaraman, L. C. Misal Castro, É. Rochette, F.-G. Fontaine, J. Am. Chem. Soc. 2017, 139, 14714–14723.
- 55M.-A. Légaré, É. Rochette, J. Légaré Lavergne, N. Bouchard, F.-G. Fontaine, Chem. Commun. 2016, 52, 5387–5390.
- 56N. Bouchard, F.-G. Fontaine, Dalton Trans. 2019, 48, 4846–4856.
- 57A. Jayaraman, L. C. Misal Castro, F.-G. Fontaine, Org. Process Res. Dev. 2018, 22, 1489–1499.
- 58T. Stahl, K. Müther, Y. Ohki, K. Tatsumi, M. Oestreich, J. Am. Chem. Soc. 2013, 135, 10978–10981.
- 59É. Rochette, V. Desrosiers, Y. Soltani, F.-G. Fontaine, J. Am. Chem. Soc. 2019, 141, 12305–12311.
- 60J. M. Lipshultz, Y. Fu, P. Liu, A. T. Radosevich, Chem. Sci. 2021, 12, 1031–1037.
- 61A. Escande, D. L. Crossley, J. Cid, I. A. Cade, I. Vitorica-Yrezabal, M. J. Ingleson, Dalton Trans. 2016, 45, 17160–17167.
- 62K. Mitsudo, K. Shigemori, H. Mandai, A. Wakamiya, S. Suga, Org. Lett. 2018, 20, 7336–7340.
- 63K. Matsui, S. Oda, K. Yoshiura, K. Nakajima, N. Yasuda, T. Hatakeyama, J. Am. Chem. Soc. 2018, 140, 1195–1198.
- 64N. Ikeda, S. Oda, R. Matsumoto, M. Yoshioka, D. Fukushima, K. Yoshiura, N. Yasuda, T. Hatakeyama, Adv. Mater. 2020, 32, 2004072.
- 65S. Oda, W. Kumano, T. Hama, R. Kawasumi, K. Yoshiura, T. Hatakeyama, Angew. Chem. Int. Ed. 2021, 60, 2882–2886; Angew. Chem. 2021, 133, 2918–2922.
- 66H. Tanaka, S. Oda, G. Ricci, H. Gotoh, K. Tabata, R. Kawasumi, D. Beljonne, Y. Olivier, T. Hatakeyama, Angew. Chem. Int. Ed. 2021, 60, 17910–17914; Angew. Chem. 2021, 133, 18054–18058.
- 67S. Oda, B. Kawakami, Y. Yamasaki, R. Matsumoto, M. Yoshioka, D. Fukushima, S. Nakatsuka, T. Hatakeyama, J. Am. Chem. Soc. 2022, 144, 106–112.
- 68J. S. McGough, J. Cid, M. J. Ingleson, Chem. Eur. J. 2017, 23, 8180–8184.
- 69Q. Zhong, S. Qin, Y. Yin, J. Hu, H. Zhang, Angew. Chem. Int. Ed. 2018, 57, 14891–14895; Angew. Chem. 2018, 130, 15107–15111.
- 70Y. Zou, B. Zhang, L. Wang, H. Zhang, Org. Lett. 2021, 23, 2821–2825.
- 71M. J. S. Dewar, V. P. Kubba, R. Pettit, J. Chem. Soc. 1958, 3073–3076.
- 72M. J. S. Dewar, R. Dietz, Tetrahedron Lett. 1959, 1, 21–23.
10.1016/S0040-4039(01)99449-3 Google Scholar
- 73M. J. S. Dewar, R. Dietz, J. Chem. Soc. 1959, 2728–2730.
- 74M. J. S. Dewar, C. Kaneko, M. K. Bhattacharjee, J. Am. Chem. Soc. 1962, 84, 4884–4887.
- 75M. J. S. Dewar, W. H. Poesche, J. Org. Chem. 1964, 29, 1757–1762.
- 76F. A. Davis, M. J. S. Dewar, J. Am. Chem. Soc. 1968, 90, 3511–3515.
- 77H. Laaziri, L. O. Bromm, F. Lhermitte, R. M. Gschwind, P. Knochel, J. Am. Chem. Soc. 1999, 121, 6940–6941.
- 78J. A. Varela, D. Peña, B. Goldfuss, K. Polborn, P. Knochel, Org. Lett. 2001, 3, 2395–2398.
- 79J. A. Varela, D. Peña, B. Goldfuss, D. Denisenko, J. Kulhanek, K. Polborn, P. Knochel, Chem. Eur. J. 2004, 10, 4252–4264.
- 80Q. J. Zhou, K. Worm, R. E. Dolle, J. Org. Chem. 2004, 69, 5147–5149.
- 81R. L. Letsinger, D. B. Maclean, J. Am. Chem. Soc. 1963, 85, 2230–2236.
- 82T. S. De Vries, A. Prokofjevs, J. N. Harvey, E. Vedejs, J. Am. Chem. Soc. 2009, 131, 14679–14687.
- 83A. Prokofjevs, J. Jermaks, A. Borovika, J. W. Kampf, E. Vedejs, Organometallics 2013, 32, 6701–6711.
- 84J. Zhang, H. Jung, D. Kim, S. Park, S. Chang, Angew. Chem. Int. Ed. 2019, 58, 7361–7365; Angew. Chem. 2019, 131, 7439–7443.
- 85N. Ishida, T. Moriya, T. Goya, M. Murakami, J. Org. Chem. 2010, 75, 8709–8712.
- 86Z. Zhao, Z. Chang, B. He, B. Chen, C. Deng, P. Lu, H. Qiu, B. Z. Tang, Chem. Eur. J. 2013, 19, 11512–11517.
- 87J. Chen, R. A. Lalancette, F. Jäkle, Organometallics 2013, 32, 5843–5851.
- 88M. Yusuf, K. Liu, F. Guo, R. A. Lalancette, F. Jäkle, Dalton Trans. 2016, 45, 4580–4587.
- 89K. Liu, R. A. Lalancette, F. Jäkle, J. Am. Chem. Soc. 2017, 139, 18170–18173.
- 90S. A. Iqbal, K. Yuan, J. Cid, J. Pahl, M. J. Ingleson, Org. Biomol. Chem. 2021, 19, 2949–2958.
- 91A. C. Shaikh, D. S. Ranade, S. Thorat, A. Maity, P. P. Kulkarni, R. G. Gonnade, P. Munshi, N. T. Patil, Chem. Commun. 2015, 51, 16115–16118.
- 92D. L. Crossley, I. A. Cade, E. R. Clark, A. Escande, M. J. Humphries, S. M. King, I. Vitorica-Yrezabal, M. J. Ingleson, M. L. Turner, Chem. Sci. 2015, 6, 5144–5151.
- 93D. L. Crossley, I. Vitorica-Yrezabal, M. J. Humphries, M. L. Turner, M. J. Ingleson, Chem. Eur. J. 2016, 22, 12439–12448.
- 94D. L. Crossley, L. Urbano, R. Neumann, S. Bourke, J. Jones, L. A. Dailey, M. Green, M. J. Humphries, S. M. King, M. L. Turner, M. J. Ingleson, ACS Appl. Mater. Interfaces 2017, 9, 28243–28249.
- 95B. P. Dash, I. Hamilton, D. J. Tate, D. L. Crossley, J.-S. Kim, M. J. Ingleson, M. L. Turner, J. Mater. Chem. C 2019, 7, 718–724.
- 96M. Shigeno, M. Imamatsu, Y. Kai, M. Kiriyama, S. Ishida, K. Nozawa-Kumada, Y. Kondo, Org. Lett. 2021, 23, 8023–8027.
- 97S. Oda, H. Abe, N. Yasuda, T. Hatakeyama, Chem. Asian J. 2019, 14, 1657–1661.
- 98H. Gotoh, S. Nakatsuka, H. Tanaka, N. Yasuda, Y. Haketa, H. Maeda, T. Hatakeyama, Angew. Chem. Int. Ed. 2021, 60, 12835–12840; Angew. Chem. 2021, 133, 12945–12950.
- 99O. Sadek, A. Le Gac, N. Hidalgo, S. Mallet-Ladeira, K. Miqueu, G. Bouhadir, D. Bourissou, Angew. Chem. Int. Ed. 2022, 61, e202110102; Angew. Chem. 2022, 134, e202110102.
- 100J. Lv, X.-J. Zhang, M. Wang, Y. Zhao, Z. Shi, Chem. Eur. J. 2022, 28, e202104100.
- 101L. Niu, H. Yang, R. Wang, H. Fu, Org. Lett. 2012, 14, 2618–2621.
- 102G. Wu, B. Pang, Y. Wang, L. Yan, L. Chen, T. Ma, Y. Ji, J. Org. Chem. 2021, 86, 5933–5942.
- 103S. Rej, A. Das, N. Chatani, Chem. Sci. 2021, 12, 11447–11454.
- 104G. Wu, X. Xu, S. Wang, L. Chen, B. Pang, T. Ma, Y. Ji, Chin. Chem. Lett. 2022, 33, 2005–2008.
- 105K. Yang, G. Zhang, Q. Song, Chem. Sci. 2018, 9, 7666–7672.
- 106C. Cazorla, T. S. De Vries, E. Vedejs, Org. Lett. 2013, 15, 984–987.
- 107S. A. Iqbal, J. Cid, R. J. Procter, M. Uzelac, K. Yuan, M. J. Ingleson, Angew. Chem. Int. Ed. 2019, 58, 15381–15385; Angew. Chem. 2019, 131, 15525–15529.
- 108J. Lv, X. Chen, X.-S. Xue, B. Zhao, Y. Liang, M. Wang, L. Jin, Y. Yuan, Y. Han, Y. Zhao, Y. Lu, J. Zhao, W.-Y. Sun, K. N. Houk, Z. Shi, Nature 2019, 575, 336–341.
- 109J. Lv, B. Zhao, Y. Yuan, Y. Han, Z. Shi, Nat. Commun. 2020, 11, 1316.
- 110G. Wu, X. Fu, Y. Wang, K. Deng, L. Zhang, T. Ma, Y. Ji, Org. Lett. 2020, 22, 7003–7007.
- 111Z.-J. Wang, X. Chen, L. Wu, J. J. Wong, Y. Liang, Y. Zhao, K. N. Houk, Z. Shi, Angew. Chem. Int. Ed. 2021, 60, 8500–8504; Angew. Chem. 2021, 133, 8581–8585.
- 112S. Rej, N. Chatani, J. Am. Chem. Soc. 2021, 143, 2920–2929.
- 113K. Yamazaki, S. Rej, Y. Ano, N. Chatani, Org. Lett. 2022, 24, 213–217.
- 114J. Pahl, E. Noone, M. Uzelac, K. Yuan, M. J. Ingleson, Angew. Chem. Int. Ed. 2022, 61, e202206230; Angew. Chem. 2022, 134, e202206230.
- 115S. Oda, K. Ueura, B. Kawakami, T. Hatakeyama, Org. Lett. 2020, 22, 700–704.
- 116A. Osi, D. Mahaut, N. Tumanov, L. Fusaro, J. Wouters, B. Champagne, A. Chardon, G. Berionni, Angew. Chem. Int. Ed. 2022, 61, e202112342; Angew. Chem. 2022, 134, e202112342.