Oxidative Radical NHC Catalysis: Divergent Difunctionalization of Olefins through Intermolecular Hydrogen Atom Transfer
Qing-Zhu Li
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
These authors contributed equally to this work.
Search for more papers by this authorYan-Qing Liu
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
These authors contributed equally to this work.
Search for more papers by this authorXin-Xin Kou
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
Search for more papers by this authorWen-Lin Zou
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
Search for more papers by this authorTing Qi
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
Search for more papers by this authorPeng Xiang
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
Search for more papers by this authorJin-Dun Xing
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
Search for more papers by this authorXiang Zhang
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
Search for more papers by this authorCorresponding Author
Jun-Long Li
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
Search for more papers by this authorQing-Zhu Li
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
These authors contributed equally to this work.
Search for more papers by this authorYan-Qing Liu
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
These authors contributed equally to this work.
Search for more papers by this authorXin-Xin Kou
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
Search for more papers by this authorWen-Lin Zou
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
Search for more papers by this authorTing Qi
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
Search for more papers by this authorPeng Xiang
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
Search for more papers by this authorJin-Dun Xing
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
Search for more papers by this authorXiang Zhang
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
Search for more papers by this authorCorresponding Author
Jun-Long Li
Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu, 610106 China
Search for more papers by this authorGraphical Abstract
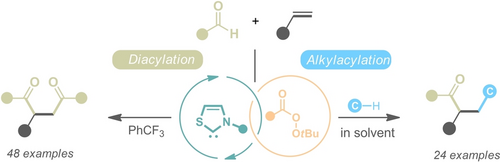
An unprecedented oxidative radical NHC catalytic system is reported that allows an efficient difunctionalization of olefins for the divergent synthesis of various ketones. Key to success is the use of peroxyesters as “dual-role” reagents to achieve a sequential single-electron oxidation and intermolecular hydron atom transfer.
Abstract
Oxidative N-heterocyclic carbene (NHC) organocatalysis, typically leading to the formation of acyl azolium reactive intermediates, constitutes one of the most important activation strategies for the NHC-catalyzed chemical transformations. Here, we report an unprecedented oxidative radical NHC catalysis by using peroxyester as the external single-electron oxidant to realize divergent difunctionalization of olefins. The key to success of this chemistry is the catalytic generation of oxygen radicals that could trigger an intermolecular hydrogen atom transfer to activate the inert C−H bonds, thereby enabling the productive radical relay process. With this protocol, commonly used general chemicals could serve as radical precursors to allow efficient synthesis of value-added products in a straightforward and cost-effective manner. Preliminary mechanistic investigations, including control experiments and DFT calculations, shed light on the NHC organocatalytic radical reaction mechanism.
Conflict of interest
The authors declare the following competing financial interest(s): A patent application (CN113336721A) based on this work has been filed by Chengdu University.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202207824-sup-0001-4p.cif325.2 KB | Supporting Information |
| anie202207824-sup-0001-8s.cif1.4 MB | Supporting Information |
| anie202207824-sup-0001-misc_information.pdf15.3 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. N. Hopkinson, C. Richter, M. Schedler, F. Glorius, Nature 2014, 510, 485–496;
- 1bD. M. Flanigan, F. Romanov-Michailidis, N. A. White, T. Rovis, Chem. Rev. 2015, 115, 9307–9387;
- 1cR. S. Menon, A. T. Biju, V. Nair, Chem. Soc. Rev. 2015, 44, 5040–5052;
- 1dK. J. R. Murauski, A. A. Jaworski, K. A. Scheidt, Chem. Soc. Rev. 2018, 47, 1773–1782;
- 1eX.-Y. Chen, Z.-H. Gao, S. Ye, Acc. Chem. Res. 2020, 53, 690–702;
- 1fH. Ohmiya, ACS Catal. 2020, 10, 6862–6869.
- 2For the works on the existence, isolation and properties of Breslow intermediates, see:
- 2aA. Berkessel, V. R. Yatham, S. Elfert, J.-M. Neudörfl, Angew. Chem. Int. Ed. 2013, 52, 11158–11162; Angew. Chem. 2013, 125, 11364–11369;
- 2bM. Paul, P. Sudkaow, A. Wessels, N. E. Schlörer, J.-M. Neudörfl, A. Berkessel, Angew. Chem. Int. Ed. 2018, 57, 8310–8315; Angew. Chem. 2018, 130, 8443–8448;
- 2cM. Paul, J.-M. Neudörfl, A. Berkessel, Angew. Chem. Int. Ed. 2019, 58, 10596–10600; Angew. Chem. 2019, 131, 10706–10710;
- 2dA. Biswas, J.-M. Neudörfl, N. E. Schlörer, A. Berkessel, Angew. Chem. Int. Ed. 2021, 60, 4507–4511; Angew. Chem. 2021, 133, 4557–4561;
- 2eM. Paul, M. Breugst, J.-M. Neudörfl, R. B. Sunoj, A. Berkessel, J. Am. Chem. Soc. 2016, 138, 5044–5051.
- 3
- 3aC. E. I. Knappke, A. Imami, A. J. von Wangelin, ChemCatChem 2012, 4, 937–941;
- 3bH. U. Vora, P. Wheeler, T. Rovis, Adv. Synth. Catal. 2012, 354, 1617–1639;
- 3cS. De Sarkar, A. Biswas, R. C. Samanta, A. Studer, Chem. Eur. J. 2013, 19, 4664–4678;
- 3dO. A. Tomashenko, V. V. Grushin, Chem. Rev. 2011, 111, 4475–4521;
- 3eT. Pavithra, E. S. Devi, C. U. Maheswari, Asian J. Org. Chem. 2021, 10, 1861–1883.
- 4
- 4aT. Ishii, K. Nagao, H. Ohmiya, Chem. Sci. 2020, 11, 5630–5636;
- 4bL. Dai, S. Ye, Chin. Chem. Lett. 2021, 32, 660–667;
- 4cQ.-Z. Li, R. Zeng, B. Han, J.-L. Li, Chem. Eur. J. 2021, 27, 3238–3250;
- 4dK.-Q. Chen, H. Sheng, Q. Liu, P.-L. Shao, X.-Y. Chen, Sci. China Chem. 2021, 64, 7–16;
- 4eK. Zhao, D. Enders, Angew. Chem. Int. Ed. 2017, 56, 3754–3756; Angew. Chem. 2017, 129, 3808–3810;
- 4fA. Mavroskoufis, M. Jakob, M. N. Hopkinson, ChemPhotoChem 2020, 4, 5147–5153.
- 5For the mechanistic studies on the nature and redox potential of Breslow intermediate derived radicals, see:
- 5aV. Regnier, E. A. Romero, F. Molton, R. Jazzar, G. Bertrand, D. Martin, J. Am. Chem. Soc. 2019, 141, 1109–1117;
- 5bL. Delfau, S. Nichilo, F. Molton, J. Broggi, E. Tomás-Mendivil, D. Martin, Angew. Chem. Int. Ed. 2021, 60, 26783–26789; Angew. Chem. 2021, 133, 26987–26993;
- 5cJ. Rehbein, S.-M. Ruser, J. Phan, Chem. Sci. 2015, 6, 6013–6018.
- 6
- 6aJ. Guin, S. D. Sarkar, S. Grimme, A. Studer, Angew. Chem. Int. Ed. 2008, 47, 8727–8730; Angew. Chem. 2008, 120, 8855–8858;
- 6bY. Zhang, Y. Du, Z. Huang, J. Xu, X. Wu, Y. Wang, M. Wang, S. Yang, R. D. Webster, Y. R. Chi, J. Am. Chem. Soc. 2015, 137, 2416–2419;
- 6cN. A. White, T. Rovis, J. Am. Chem. Soc. 2014, 136, 14674–14677;
- 6dN. A. White, T. Rovis, J. Am. Chem. Soc. 2015, 137, 10112–10115;
- 6eW. Yang, W. Hu, X. Dong, X. Li, J. Sun, Angew. Chem. Int. Ed. 2016, 55, 15783–15786; Angew. Chem. 2016, 128, 16015–16018;
- 6fB.-S. Li, Y. Wang, R. S. J. Proctor, Y. Zhang, R. D. Webster, S. Yang, B. Song, Y. R. Chi, Nat. Commun. 2016, 7, 12933–12940;
- 6gX. Wu, Y. Zhang, Y. Wang, J. Ke, M. Jeret, R. N. Reddi, S. Yang, B.-A. Song, Y. R. Chi, Angew. Chem. Int. Ed. 2017, 56, 2942–2946; Angew. Chem. 2017, 129, 2988–2992;
- 6hT. Ishii, Y. Kakeno, K. Nagao, H. Ohmiya, J. Am. Chem. Soc. 2019, 141, 3854–3858;
- 6iA. Mavroskoufis, K. Rajes, P. Golz, A. Agrawal, V. Ruß, J. P. Götze, M. N. Hopkinson, Angew. Chem. Int. Ed. 2020, 59, 3190–3194; Angew. Chem. 2020, 132, 3216–3220;
- 6jA. V. Bay, K. P. Fitzpatrick, R. C. Betori, K. A. Scheidt, Angew. Chem. Int. Ed. 2020, 59, 9143–9148; Angew. Chem. 2020, 132, 9228–9233;
- 6kQ.-Y. Meng, L. Lezius, A. Studer, Nat. Commun. 2021, 12, 2068;
- 6lH. Huang, Q.-S. Dai, H.-J. Leng, Q.-Z. Li, S.-L. Yang, Y.-M. Tao, X. Zhang, T. Qi, J.-L. Li, Chem. Sci. 2022, 13, 2584–2590.
- 7
- 7aL. Wang, J. Xiao, Top. Curr. Chem. 2016, 374, 17;
- 7bJ. M. Mayer, Acc. Chem. Res. 2011, 44, 36–46;
- 7cL. Capaldo, D. Ravelli, Eur. J. Org. Chem. 2017, 2056–2071;
- 7dM. Salamone, M. Bietti, Acc. Chem. Res. 2015, 48, 2895–2903;
- 7eY. Matsuki, N. Ohnishi, Y. Kakeno, S. Takemoto, T. Ishii, K. Nagao, H. Ohmiya, Nat. Commun. 2021, 12, 3848;
- 7fQ.-Z. Li, R. Zeng, Y. Fan, Y.-Q. Liu, T. Qi, X. Zhang, J.-L. Li, Angew. Chem. Int. Ed. 2022, 61, e202116629; Angew. Chem. 2022, 134, e202116629;
- 7gY. Man, S. Liu, B. Xu, X. Zeng, Org. Lett. 2022, 24, 944–948.
- 8E. S. Devi, T. Pavithra, A. Tamilselvi, S. Nagarajan, V. Sridharan, C. U. Maheswari, Org. Lett. 2020, 22, 3576–3580.
- 9
- 9aT. Ishii, K. Ota, K. Nagao, H. Ohmiya, J. Am. Chem. Soc. 2019, 141, 14073–14077;
- 9bJ.-L. Li, Y.-Q. Liu, W.-L. Zou, R. Zeng, X. Zhang, Y. Liu, B. Han, Y. He, H.-J. Leng, Q.-Z. Li, Angew. Chem. Int. Ed. 2020, 59, 1863–1870; Angew. Chem. 2020, 132, 1879–1886;
- 9cQ.-Y. Meng, N. Döben, A. Studer, Angew. Chem. Int. Ed. 2020, 59, 19956–19960; Angew. Chem. 2020, 132, 20129–20134;
- 9dW. Liu, A. Vianna, Z. Zhang, S. Huang, L. Huang, M. Melaimi, G. Bertrand, X. Yan, Chem. Catal. 2021, 1, 196–206;
- 9eZ. Li, M. Huang, X. Zhang, J. Chen, Y. Huang, ACS Catal. 2021, 11, 10123–10130;
- 9fM. Kusakabe, K. Nagao, H. Ohmiya, Org. Lett. 2021, 23, 7242–7247;
- 9gY. Gao, Y. Quan, Z. Li, L. Gao, Z. Zhang, X. Zou, R. Yan, Y. Qu, K. Guo, Org. Lett. 2021, 23, 183–189;
- 9hL. Chen, S. Jin, J. Gao, T. Liu, Y. Shao, J. Feng, K. Wang, T. Lu, D. Du, Org. Lett. 2021, 23, 394–399;
- 9iI. Kim, H. Im, H. Lee, S. Hong, Chem. Sci. 2020, 11, 3192–3197;
- 9jB. Zhang, Q. Peng, D. Guo, J. Wang, Org. Lett. 2020, 22, 443–447;
- 9kH.-B. Yang, Z.-H. Wang, J.-M. Li, C. Wu, Chem. Commun. 2020, 56, 3801–3804;
- 9lK. Ota, K. Nagao, H. Ohmiya, Org. Lett. 2020, 22, 3922–3925;
- 9mS. Jin, X. Sui, G. C. Haug, V. D. Nguyen, H. T. Dang, H. D. Arman, O. V. Larionov, ACS Catal. 2022, 12, 285–294;
- 9nH. Liu, Y.-F. Han, Z.-H. Gao, C.-L. Zhang, C. Wang, S. Ye, ACS Catal. 2022, 12, 1657–1663;
- 9oM. Schedler, D.-S. Wang, F. Glorius, Angew. Chem. Int. Ed. 2013, 52, 2585–2589; Angew. Chem. 2013, 125, 2645–2649.
- 10Y. Qin, L. Zhu, S. Luo, Chem. Rev. 2017, 117, 9433–9520.
- 11For detailed optimization studies, see the Supporting Information.
- 12Deposition Numbers 2067127 (for 4 p) and 2129803 (for 8 s) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 13
- 13aL. Xu, J. Chen, L. Chu, Org. Chem. Front. 2019, 6, 512–516;
- 13bW. Sheng, C. Jin, S. Shan, Y. Jia, J. Gao, Chin. J. Org. Chem. 2016, 36, 325–329;
- 13cY. Liu, J.-L. Zhang, R.-J. Song, J.-H. Li, Org. Chem. Front. 2014, 1, 1289–1294.
- 14J. A. M. Simōes, D. Griller, Chem. Phys. Lett. 1989, 158, 175–177.
- 15J. L. Holmes, F. P. Lossing, A. Maccoll, J. Am. Chem. Soc. 1988, 110, 7339–7342.
- 16For a mechanism study on NHC-catalzyed radical transformation, see: A. V. Bay, K. P. Fitzpatrick, G. A. González-Montiel, A. O. Farah, P. H.-Y. Cheong, K. A. Scheidt, Angew. Chem. Int. Ed. 2021, 60, 17925–17931; Angew. Chem. 2021, 133, 18069–18075.
- 17
- 17aY. Zhao, D. G. Truhlar, Theor. Chem. Acc. 2008, 120, 215–241;
- 17bY. Zhao, D. G. Truhlar, Acc. Chem. Res. 2008, 41, 157–167.
- 18K. N. Houk, Acc. Chem. Res. 1975, 8, 361–369.
- 19
- 19aE. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen, W. Yang, J. Am. Chem. Soc. 2010, 132, 6498–6506;
- 19bC. Lefebvre, G. Rubez, H. Khartabil, J. C. Boisson, J. Contreras-Garcia, E. Henon, Phys. Chem. Chem. Phys. 2017, 19, 17928–17936.
- 20T. Lu, F. Chen, J. Comput. Chem. 2012, 33, 580–592.
- 21For detailed DFT studies of the whole reaction pathway, see the Supporting Information.
- 22Another possibility is that the free carbene might not be the catalytically active species, see: S. Gehrke, O. Hollóczki, Angew. Chem. Int. Ed. 2017, 56, 16395–16398; Angew. Chem. 2017, 129, 16613–16617.
- 23
- 23aY.-L. Liu, Y.-J. Ouyang, H. Zheng, H. Liu, W.-T. Wei, Chem. Commun. 2021, 57, 6111–6120;
- 23bA. Banerjee, Z. Lei, M.-Y. Ngai, Synthesis 2019, 51, 303–333.