Mild Acidosis-Directed Signal Amplification in Tumor Microenvironment via Spatioselective Recruitment of DNA Amplifiers
Dr. Zhenghan Di
CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China
College of Materials Science and Optoelectronic Technology, University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorProf. Dr. Xueguang Lu
Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139 USA
Search for more papers by this authorDr. Jian Zhao
CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China
College of Materials Science and Optoelectronic Technology, University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorProf. Ana Jaklenec
David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139 USA
Search for more papers by this authorProf. Yuliang Zhao
CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China
Search for more papers by this authorProf. Robert Langer
David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139 USA
Search for more papers by this authorCorresponding Author
Prof. Lele Li
CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China
College of Materials Science and Optoelectronic Technology, University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorDr. Zhenghan Di
CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China
College of Materials Science and Optoelectronic Technology, University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorProf. Dr. Xueguang Lu
Key Laboratory of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 China
David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139 USA
Search for more papers by this authorDr. Jian Zhao
CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China
College of Materials Science and Optoelectronic Technology, University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorProf. Ana Jaklenec
David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139 USA
Search for more papers by this authorProf. Yuliang Zhao
CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China
Search for more papers by this authorProf. Robert Langer
David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139 USA
Search for more papers by this authorCorresponding Author
Prof. Lele Li
CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China
College of Materials Science and Optoelectronic Technology, University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorGraphical Abstract
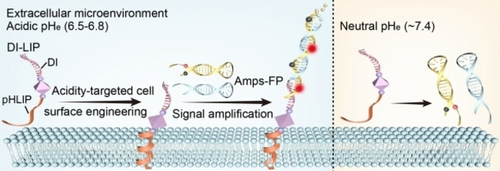
A DNA-based strategy is developed for spatially-selective amplification of acidic signals in the extracellular milieu of tumors. Amplified imaging of the tumor microenvironment with improved sensitivity was achieved by acidity-responsive engineering of the cell surface with DNA receptors for controlled recruitment of fluorescent amplifiers.
Abstract
DNA biotechnology offers intriguing opportunities for amplification-based sensitive detection. However, spatiotemporally-controlled manipulation of signal amplification for in situ imaging of the tumor microenvironment remains an outstanding challenge. Here, we demonstrate a DNA-based strategy that can spatial-selectively amplify the acidic signal in the extracellular milieu of the tumor to achieve specific imaging with improved sensitivity. The strategy, termed mild acidosis-targeted amplification (MAT-amp), leverages the specific acidic microenvironment to engineer tumor cells with artificial DNA receptors through a pH (low) insertion peptide, which permits controlled recruitment of fluorescent amplifiers via a hybridization chain reaction. The acidosis-responsive amplification cascade enables significant fluorescence enhancement in tumors with a reduced background signal in normal tissues, leading to improved signal-to-background ratio. These results highlight the utility of MAT-amp for in situ imaging of the microenvironment characterized by pH disequilibrium.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202205436-sup-0001-misc_information.pdf1.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1H. J. Adrogué, N. E. Madias, N. Engl. J. Med. 1998, 338, 26–34.
- 2S. Grinstein, C. J. Swallow, O. D. Swallow, Clin. Biochem. 1991, 24, 241–247.
- 3P. P. Hsu, D. M. Sabatini, Cell 2008, 134, 703–707.
- 4B. A. Webb, M. Chimenti, M. P. Jacobson, D. L. Barber, Nat. Rev. Cancer 2011, 11, 671–677.
- 5D. Hanahan, R. A. Weinberg, Cell 2011, 144, 646–674.
- 6C. Corbet, O. Feron, Nat. Rev. Cancer 2017, 17, 577–593.
- 7Y. J. Chen, B. Groves, R. A. Muscat, G. Seelig, Nat. Nanotechnol. 2015, 10, 748–760.
- 8S. H. Rouhanifard, I. A. Mellis, M. Dunagin, S. Bayatpour, C. L. Jiang, I. Dardani, O. Symmons, B. Emert, E. Torre, A. Cote, A. Sullivan, J. A. Stamatoyannopoulos, A. Raj, Nat. Biotechnol. 2019, 37, 84–89.
- 9P. M. Lizardi, X. Huang, Z. Zhu, P. Bray-Ward, D. C. Thomas, D. C. Ward, Nat. Genet. 1998, 19, 225–232.
- 10B. Schweitzer, S. Wiltshire, J. Lambert, S. O'Malley, K. Kukanskis, Z. Zhu, S. F. Kingsmore, P. M. Lizardi, D. C. Ward, Proc. Natl. Acad. Sci. USA 2000, 97, 10113–10119.
- 11C. Larsson, I. Grundberg, O. Söderberg, M. Nilsson, Nat. Methods 2010, 7, 395–397.
- 12M. Nagendran, D. P. Riordan, P. B. Harbury, T. J. Desai, eLife 2018, 7, e30510.
- 13X. Wang, W. E. Allen, M. A. Wright, E. L. Sylwestrak, N. Samusik, S. Vesuna, K. Evans, C. Liu, C. Ramakrishnan, J. Liu, G. P. Nolan, F. A. Bava, K. Deisseroth, Science 2018, 361, eaat5691.
- 14A. R. Connolly, M. Trau, Angew. Chem. Int. Ed. 2010, 49, 2720–2723; Angew. Chem. 2010, 122, 2780–2783.
- 15R. Duan, X. Zuo, S. Wang, X. Quan, D. Chen, Z. Chen, L. Jiang, C. Fan, F. Xia, J. Am. Chem. Soc. 2013, 135, 4604–4607.
- 16R. Freeman, X. Liu, I. Willner, Nano Lett. 2011, 11, 4456–4461.
- 17W. Xu, X. Xue, T. Li, H. Zeng, X. Liu, Angew. Chem. Int. Ed. 2009, 48, 6849–6852; Angew. Chem. 2009, 121, 6981–6984.
- 18J. Y. Lau, G. L. Davis, J. Kniffen, K. Qian, M. S. Urdea, C. Chan, M. Mizokami, P. D. Neuwald, J. C. Wilber, Lancet 1993, 341, 1501–1504.
- 19N. Battich, T. Stoeger, L. Pelkmans, Nat. Methods 2013, 10, 1127–1133.
- 20J. Y. Kishi, T. E. Schaus, N. Gopalkrishnan, F. Xuan, P. Yin, Nat. Chem. 2018, 10, 155–164.
- 21J. Y. Kishi, S. W. Lapan, B. J. Beliveau, E. R. West, A. Zhu, H. M. Sasaki, S. K. Saka, Y. Wang, C. L. Cepko, P. Yin, Nat. Methods 2019, 16, 533–544.
- 22R. M. Dirks, N. A. Pierce, Proc. Natl. Acad. Sci. USA 2004, 101, 15275–15278.
- 23H. M. T. Choi, J. Y. Chang, L. A. Trinh, J. E. Padilla, S. E. Fraser, N. A. Pierce, Nat. Biotechnol. 2010, 28, 1208–1212.
- 24S. Venkataraman, R. M. Dirks, P. W. Rothemund, E. Winfree, N. A. Pierce, Nat. Nanotechnol. 2007, 2, 490–494.
- 25Z. Cheglakov, T. M. Cronin, C. He, Y. Weizmann, J. Am. Chem. Soc. 2015, 137, 6116–6119.
- 26H. Chu, J. Zhao, Y. Mi, Y. Zhao, L. Li, Angew. Chem. Int. Ed. 2019, 58, 14877–14881; Angew. Chem. 2019, 131, 15019–15023.
- 27Z. Qing, J. Xu, J. Hu, J. Zheng, L. He, Z. Zou, S. Yang, W. Tan, R. Yang, Angew. Chem. Int. Ed. 2019, 58, 11574–11585; Angew. Chem. 2019, 131, 11698–11709.
- 28R. Lin, Q. Feng, P. Li, P. Zhou, R. Wang, Z. Liu, Z. Wang, X. Qi, N. Tang, F. Shao, M. Luo, Nat. Methods 2018, 15, 275–278.
- 29S. K. Saka, Y. Wang, J. Y. Kishi, A. Zhu, Y. Zeng, W. Xie, K. Kirli, C. Yapp, M. Cicconet, B. J. Beliveau, S. W. Lapan, S. Yin, M. Lin, E. S. Boyden, P. S. Kaeser, G. Pihan, G. M. Church, P. Yin, Nat. Biotechnol. 2019, 37, 1080–1090.
- 30G. C. Gavins, K. Gröger, M. D. Bartoschek, P. Wolf, A. G. Beck-Sickinger, S. Bultmann, O. Seitz, Nat. Chem. 2021, 13, 15–23.
- 31O. A. Andreev, A. D. Dupuy, M. Segala, S. Sandugu, D. A. Serra, C. O. Chichester, D. M. Engelman, Y. K. Reshetnyak, Proc. Natl. Acad. Sci. USA 2007, 104, 7893–7898.
- 32C. J. Cheng, R. Bahal, I. A. Babar, Z. Pincus, F. Barrera, C. Liu, A. Svoronos, D. T. Braddock, P. M. Glazer, D. M. Engelman, W. M. Saltzman, F. J. Slack, Nature 2015, 518, 107–110.
- 33L. C. Wyatt, A. Moshnikova, T. Crawford, D. M. Engelman, O. A. Andreev, Y. K. Reshetnyak, Proc. Natl. Acad. Sci. USA 2018, 115, E2811–E2818.
- 34Z. Di, J. Zhao, H. Chu, W. Xue, Y. Zhao, L. Li, Adv. Mater. 2019, 31, 1901885.
- 35J. Golijanin, A. Amin, A. Moshnikova, J. M. Brito, T. Y. Tran, R.-C. Adochite, G. O. Andreev, T. Crawford, D. M. Engelman, O. A. Andreev, Y. K. Reshetnyak, D. Golijanin, Proc. Natl. Acad. Sci. USA 2016, 113, 11829–11834.
- 36T. T. Tapmeier, A. Moshnikova, J. Beech, D. Allen, P. Kinchesh, S. Smart, A. Harris, A. McIntyre, D. M. Engelman, O. A. Andreev, Y. K. Reshetnyak, R. J. Muschel, Proc. Natl. Acad. Sci. USA 2015, 112, 9710–9715.
- 37L. C. Wyatt, J. S. Lewis, O. A. Andreev, Y. K. Reshetnyak, D. M. Engelman, Trends Biotechnol. 2017, 35, 653–664.
- 38G. Zhu, J. Zheng, E. Song, M. Donovan, K. Zhang, C. Liu, W. Tan, Proc. Natl. Acad. Sci. USA 2013, 110, 7998–8003.
- 39X. Zheng, H. Mao, D. Huo, W. Wu, B. Liu, X. Jiang, Nat. Biomed. Eng. 2017, 1, 0057.
- 40K. Polyak, J. Clin. Invest. 2011, 121, 3786–3788.
- 41S. Paik, J. Bryant, E. Tan-Chiu, G. Yothers, C. Park, D. L. Wickerham, N. Wolmark, J. Natl. Cancer Inst. 2000, 92, 1991–1998.
- 42T. W. Jacobs, A. M. Gown, H. Yaziji, M. J. Barnes, S. J. Schnitt, Am. J. Clin. Pathol. 2000, 113, 251–258.
- 43P. Mi, D. Kokuryo, H. Cabral, H. Wu, Y. Terada, T. Saga, I. Aoki, N. Nishiyama, K. Kataoka, Nat. Nanotechnol. 2016, 11, 724–730.
- 44J. H. Jeong, J. J. Schmidt, R. E. Kohman, A. T. Zill, R. J. De-Volder, C. E. Smith, M. Lai, A. Shkumatov, T. W. Jensen, L. G. Schook, S. C. Zimmerman, H. Kong, J. Am. Chem. Soc. 2013, 135, 8770–8773.
- 45J. Park, B. Andrade, Y. Seo, M. J. Kim, S. C. Zimmerman, H. Kong, Chem. Rev. 2018, 118, 1664–1690.
- 46J. A. Prescher, D. H. Dube, C. R. Bertozzi, Nature 2004, 430, 873–877.
- 47H. Wang, R. Wang, K. Cai, H. He, Y. Liu, J. Yen, J. Wang, M. Xu, Y. Sun, X. Zhou, Q. Yin, L. Tang, I. T. Dobrucki, L. W. Dobrucki, E. J. Chaney, S. A. Boppart, T. Fan, S. Lezmi, X. Chen, L. Yin, J. Cheng, Nat. Chem. Biol. 2017, 13, 415–424.
- 48H. Wang, M. C. Sobral, D. K. Y. Zhang, A. N. Cartwright, A. Li, M. O. Dellacherie, C. M. Tringides, S. T. Koshy, K. W. Wu-cherpfennig, D. J. Mooney, Nat. Mater. 2020, 19, 1244–1252.