Total Synthesis of Complex Peptidyl Nucleoside Antibiotics: Asymmetric De Novo Syntheses of Miharamycin B and Its Biosynthetic Precursor
Dr. Wenjun Huang
State Key Laboratory of Applied Organic Chemistry, Department of Chemistry and School of Pharmacy, Lanzhou University, Lanzhou, 730000 P. R. China
Search for more papers by this authorDr. Shuai Fan
State Key Laboratory of Applied Organic Chemistry, Department of Chemistry and School of Pharmacy, Lanzhou University, Lanzhou, 730000 P. R. China
Search for more papers by this authorDr. Jiahui Gao
State Key Laboratory of Applied Organic Chemistry, Department of Chemistry and School of Pharmacy, Lanzhou University, Lanzhou, 730000 P. R. China
Search for more papers by this authorProf. Dr. Shangwen Luo
State Key Laboratory of Applied Organic Chemistry, Department of Chemistry and School of Pharmacy, Lanzhou University, Lanzhou, 730000 P. R. China
Search for more papers by this authorProf. Dr. Shouchu Tang
State Key Laboratory of Applied Organic Chemistry, Department of Chemistry and School of Pharmacy, Lanzhou University, Lanzhou, 730000 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jian Liu
State Key Laboratory of Applied Organic Chemistry, Department of Chemistry and School of Pharmacy, Lanzhou University, Lanzhou, 730000 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaolei Wang
State Key Laboratory of Applied Organic Chemistry, Department of Chemistry and School of Pharmacy, Lanzhou University, Lanzhou, 730000 P. R. China
State Key Laboratory of Veterinary Etiological Biology, College of Veterinary Medicine, Lanzhou University, Lanzhou, 730000 P. R. China
Search for more papers by this authorDr. Wenjun Huang
State Key Laboratory of Applied Organic Chemistry, Department of Chemistry and School of Pharmacy, Lanzhou University, Lanzhou, 730000 P. R. China
Search for more papers by this authorDr. Shuai Fan
State Key Laboratory of Applied Organic Chemistry, Department of Chemistry and School of Pharmacy, Lanzhou University, Lanzhou, 730000 P. R. China
Search for more papers by this authorDr. Jiahui Gao
State Key Laboratory of Applied Organic Chemistry, Department of Chemistry and School of Pharmacy, Lanzhou University, Lanzhou, 730000 P. R. China
Search for more papers by this authorProf. Dr. Shangwen Luo
State Key Laboratory of Applied Organic Chemistry, Department of Chemistry and School of Pharmacy, Lanzhou University, Lanzhou, 730000 P. R. China
Search for more papers by this authorProf. Dr. Shouchu Tang
State Key Laboratory of Applied Organic Chemistry, Department of Chemistry and School of Pharmacy, Lanzhou University, Lanzhou, 730000 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jian Liu
State Key Laboratory of Applied Organic Chemistry, Department of Chemistry and School of Pharmacy, Lanzhou University, Lanzhou, 730000 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaolei Wang
State Key Laboratory of Applied Organic Chemistry, Department of Chemistry and School of Pharmacy, Lanzhou University, Lanzhou, 730000 P. R. China
State Key Laboratory of Veterinary Etiological Biology, College of Veterinary Medicine, Lanzhou University, Lanzhou, 730000 P. R. China
Search for more papers by this authorGraphical Abstract
Abstract
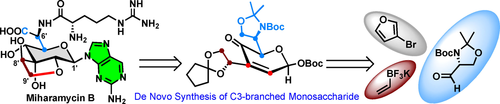
Miharamycins belong to a class of peptidyl nucleoside antibiotics with a unique nine-carbon pyranosyl amino acid core and a rare 2-aminopurine moiety. Herein, we report the de novo total synthesis of miharamycin B and its biosynthetic precursor from 3-bromofuran and Garner's aldehyde through a modified Achmatowicz reaction. Many challenges were resolved toward the de novo synthesis of miharamycin B, including the introduction of a dense array of functional groups, the stereoselective construction of consecutive stereocenters, dealing with the variability of the anomeric positions, and promoting site-selectivity in the cyclization to form the tetrahydrofuran ring. This de novo synthesis strategy enables efficient preparation of 3′-substituted saccharides, allowing the study of their structure–activity relationships and mode of action, and meets the growing demand for the development of novel antibiotics inspired by miharamycin natural products.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202204907-sup-0001-misc_information.pdf10.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. T. Walsh, W. Zhang, ACS Chem. Biol. 2011, 6, 1000–1007.
- 2S. Santajit, N. Indrawattana, BioMed Res. Int. 2016, 2016, 2475067.
- 3
- 3aT. Noguchi, Y. Yasuda, T. Niida, T. Shomura, Ann. Phytopath. Soc. Jpn. 1968, 34, 323–327;
- 3bT. Tsuruoka, H. Yumoto, N. Ezaki, T. Niida, Sci. Rep. Meiji Seika Kenkyu Nenpo 1967, 9, 1–4;
- 3cT. Iwasa, T. Kishi, K. Matsuura, O. Wakae, J. Antibiot. 1977, 30, 1–10;
- 3dS. Harada, T. Kishi, J. Antibiot. 1977, 30, 11–16;
- 3eT. Shomura, K. Hamamoto, T. Ohashi, S. Amano, J. Yoshida, C. Moriyama, T. Niida, Sci. Rep. Meiji Seika Kenkyu Nenpo 1967, 9, 5.
- 4
- 4aS. Czernecki, S. Franco, S. Horns, J. M. Valéry, Tetrahedron Lett. 1996, 37, 4003–4006;
- 4bS. Czernecki, S. Horns, J. M. Valery, J. Org. Chem. 1995, 60, 650–655;
- 4cA. Rauter, M. Ferreira, C. Borges, T. Duarte, F. Piedade, M. Silva, H. Santos, Carbohydr. Res. 2000, 325, 1–15;
- 4dA. P. Rauter, O. Oliveira, T. Canda, E. Leroi, H. Ferreira, M. J. Ferreira, J. A. Ascenso, J. Carbohydr. Chem. 2002, 21, 257–273;
- 4eM. Etheve-Quelquejeu, J. Xie, J. M. Valéry, Synlett 2004, 1089–1091;
- 4fV. Cachatra, A. Almeida, J. Sardinha, S. D. Lucas, A. Gomes, P. D. Vaz, M. H. Florêncio, R. Nunes, D. Vila-Viçosa, M. J. Calhorda, A. P. Rauter, Org. Lett. 2015, 17, 5622–5625;
- 4gF. Marcelo, R. Abou-Jneid, M. Sollogoub, J. Marrot, A. P. Rauter, Y. Bleriot, Synlett 2009, 1269–1272.
- 5F. Marcelo, J. Jimenez-Barbero, J. Marrot, A. Rauter, P. Sinaÿ, Y. Blériot, Chem. Eur. J. 2008, 14, 10066–10073.
- 6
- 6aS. Wang, Q. Zhang, Y. Zhao, J. Sun, W. Kang, F. Wang, H. Pan, G. Tang, B. Yu, Angew. Chem. Int. Ed. 2019, 58, 10558–10562; Angew. Chem. 2019, 131, 10668–10672;
- 6bS. Wang, J. Sun, Q. Zhang, X. Cao, Y. Zhao, G. Tang, B. Yu, Angew. Chem. Int. Ed. 2018, 57, 2884–2888; Angew. Chem. 2018, 130, 2934–2938.
- 7F. Wang, W. H. Zhang, J. Zhao, W. J. Kang, S. Wang, B. Yu, H. X. Pan, G. L. Tang, J. Am. Chem. Soc. 2020, 142, 5996–6000.
- 8A. J. Romo, T. Shiraishi, H. Ikeuchi, G. M. Lin, Y. J. Geng, Y. H. Lee, P. H. Liem, T. Ma, Y. Ogasawara, K. Shin-ya, M. Nishiyama, T. Kuzuyama, H. W. Liu, J. Am. Chem. Soc. 2019, 141, 14152–14159.
- 9P. Garner, J. M. Park, Org. Synth. 2003, 70, 18.
- 10D. Niu, S. L. Buchwald, J. Am. Chem. Soc. 2015, 137, 9716–9721.
- 11K. B. Sharpless, W. Amberg, Y. L. Bennani, G. A. Crispino, J. Hartung, K. S. Jeong, H. L. Kwong, K. Morikawa, Z. M. Wang, D. Q. Xu, X. L. Zhang, J. Org. Chem. 1992, 57, 2768–2771.
- 12
- 12aF. A. J. Meskens, Synthesis 1981, 501–522;
- 12bE. H. Cordes, H. G. Bull, Chem. Rev. 1974, 74, 581–603.
- 13
- 13aA. K. Ghosh, Z. H. Chen, Org. Lett. 2013, 15, 5088–5091;
- 13bY. Ma, G. A. O'Doherty, Org. Lett. 2015, 17, 5280–5283;
- 13cR. S. Babu, Q. Chen, S. W. Kang, M. Zhou, G. A. O'Doherty, J. Am. Chem. Soc. 2012, 134, 11952–11955;
- 13dK. C. Nicolaou, R. J. Aversa, J. Jin, F. Rivas, J. Am. Chem. Soc. 2010, 132, 6855–6861;
- 13eJ. Liu, J. Wu, J. H. Fan, X. Yan, G. Mei, C. C. Li, J. Am. Chem. Soc. 2018, 140, 5365–5369;
- 13fZ. Li, F. C. Ip, N. Y. Ip, R. Tong, Chem. Eur. J. 2015, 21, 11152–11157.
- 14M. Zhou, G. A. O'Doherty, Org. Lett. 2006, 8, 4339–4342.
- 15R. S. Babu, G. A. O'Doherty, J. Carbohydr. Chem. 2005, 24, 169–177.
- 16
- 16aR. S. Babu, G. A. O'Doherty, J. Am. Chem. Soc. 2003, 125, 12406–12407;
- 16bH. Guo, G. A. O'Doherty, Angew. Chem. Int. Ed. 2007, 46, 5206–5208; Angew. Chem. 2007, 119, 5298–5300;
- 16cQ. Chen, Y. Zhong, G. A. O'Doherty, Chem. Commun. 2013, 49, 6806–6808;
- 16dS. R. Guppi, M. Zhou, G. A. O'Doherty, Org. Lett. 2006, 8, 293–296;
- 16eB. M. Trost, M. J. Krische, V. Berl, E. M. Grenzer, Org. Lett. 2002, 4, 2005–2008;
- 16fB. M. Trost, Z. P. Shi, J. Am. Chem. Soc. 1996, 118, 3037–3038;
- 16gB. M. Trost, R. Madsen, S. D. Guile, A. E. H. Elia, Angew. Chem. Int. Ed. Engl. 1996, 35, 1569–1572; Angew. Chem. 1996, 108, 1666–1668;
- 16hB. M. Trost, R. Madsen, S. D. Guile, Tetrahedron Lett. 1997, 38, 1707–1710.
- 17P. H. J. Carlsen, T. Katsuki, V. S. Martin, K. B. Sharpless, J. Org. Chem. 1981, 46, 3936–3938.
- 18Deposition Numbers 2144670 (for 12), 2144674 (for 7) and 2144672 (for 16) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.