Fluorination Triggers Fluoroalkylation: Nucleophilic Perfluoro-tert-butylation with 1,1-Dibromo-2,2-bis(trifluoromethyl)ethylene (DBBF) and CsF
Dr. Qian Wang
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling-Ling Road, Shanghai, 200032 China
Search for more papers by this authorQuan Tao
State Key Laboratory of Functional Materials of Informatics, Shanghai Institute of Microsystem and Information Technology (SIMIT), Chinese Academy of Sciences (CAS), Shanghai, 200050 China
CAS Center for ExcelleNce in Superconducting Electronics (CENSE), Chinese Academy of Sciences (CAS), Shanghai, 200050 China
Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences (UCAS), Beijing, 100049 China
Search for more papers by this authorDr. Hui Dong
State Key Laboratory of Functional Materials of Informatics, Shanghai Institute of Microsystem and Information Technology (SIMIT), Chinese Academy of Sciences (CAS), Shanghai, 200050 China
CAS Center for ExcelleNce in Superconducting Electronics (CENSE), Chinese Academy of Sciences (CAS), Shanghai, 200050 China
Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences (UCAS), Beijing, 100049 China
Search for more papers by this authorDr. Chuanfa Ni
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling-Ling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Xiaoming Xie
State Key Laboratory of Functional Materials of Informatics, Shanghai Institute of Microsystem and Information Technology (SIMIT), Chinese Academy of Sciences (CAS), Shanghai, 200050 China
CAS Center for ExcelleNce in Superconducting Electronics (CENSE), Chinese Academy of Sciences (CAS), Shanghai, 200050 China
Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences (UCAS), Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Prof. Jinbo Hu
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling-Ling Road, Shanghai, 200032 China
Search for more papers by this authorDr. Qian Wang
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling-Ling Road, Shanghai, 200032 China
Search for more papers by this authorQuan Tao
State Key Laboratory of Functional Materials of Informatics, Shanghai Institute of Microsystem and Information Technology (SIMIT), Chinese Academy of Sciences (CAS), Shanghai, 200050 China
CAS Center for ExcelleNce in Superconducting Electronics (CENSE), Chinese Academy of Sciences (CAS), Shanghai, 200050 China
Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences (UCAS), Beijing, 100049 China
Search for more papers by this authorDr. Hui Dong
State Key Laboratory of Functional Materials of Informatics, Shanghai Institute of Microsystem and Information Technology (SIMIT), Chinese Academy of Sciences (CAS), Shanghai, 200050 China
CAS Center for ExcelleNce in Superconducting Electronics (CENSE), Chinese Academy of Sciences (CAS), Shanghai, 200050 China
Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences (UCAS), Beijing, 100049 China
Search for more papers by this authorDr. Chuanfa Ni
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling-Ling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Xiaoming Xie
State Key Laboratory of Functional Materials of Informatics, Shanghai Institute of Microsystem and Information Technology (SIMIT), Chinese Academy of Sciences (CAS), Shanghai, 200050 China
CAS Center for ExcelleNce in Superconducting Electronics (CENSE), Chinese Academy of Sciences (CAS), Shanghai, 200050 China
Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences (UCAS), Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Prof. Jinbo Hu
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling-Ling Road, Shanghai, 200032 China
Search for more papers by this authorGraphical Abstract
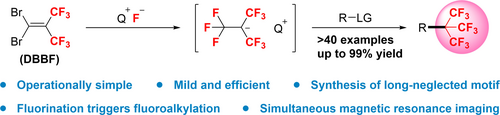
A new perfluoro-tert-butylation protocol debuts: Consecutive triple fluorinations of 1,1-dibromo-2,2-bis(trifluoromethyl)ethylene (DBBF) generate (CF3)3C− species, which enables efficient nucleophilic perfluoro-tert-butylation of various electrophiles. The C(CF3)3-containing products can be successfully applied in 19F-magnetic resonance imaging.
Abstract
Perfluoro-tert-butylation reaction has long remained a challenging task. We now report the use of 1,1-dibromo-2,2-bis(trifluoromethyl)ethylene (DBBF) as a practical reagent for perfluoro-tert-butylation reactions for the first time. Through a consecutive triple-fluorination process with DBBF and CsF, the (CF3)3C− species can be liberated and observed, which is able to serve as a robust nucleophilic perfluoro-tert-butylating agent for various electrophiles. The power of this synthetic protocol is evidenced by the efficient synthesis of structurally diverse perfluoro-tert-butylated molecules. Multiple applications demonstrate the practicability of this method, as well as the superiority of perfluoro-tert-butylated compounds as sensitive probes. The perfluoro-tert-butylated product was successfully applied in 1H- and 19F-magnetic resonance imaging (MRI) experiment with an ultra-low field (ULF) MRI system.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202113727-sup-0001-misc_information.pdf6.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aB. Ameduri, H. Sawada, Fluorinated Polymers, Royal Society of Chemistry, Cambridge, 2016;
- 1bD. Cahard, J.-A. Ma, Emerging Fluorinated Motifs: Synthesis Properties, and Applications, Wiley-VCH, Weinheim, 2020;
10.1002/9783527824342 Google Scholar
- 1cS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320–330;
- 1dR. Berger, G. Resnati, P. Metrangolo, E. Weber, J. Hulliger, Chem. Soc. Rev. 2011, 40, 3496–3508.
- 2
- 2aM. Inoue, Y. Sumii, N. Shibata, ACS Omega 2020, 5, 10633–10640;
- 2bY. Ogawa, E. Tokunaga, O. Kobayashi, K. Hirai, N. Shibata, iScience 2020, 23, 101467.
- 3S. Dandapani, L. A. Marcaurelle, Nat. Chem. Biol. 2010, 6, 861–863.
- 4
- 4aC. Alonso, E. M. Marigorta, G. Rubiales, F. Palacios, Chem. Rev. 2015, 115, 1847–1935;
- 4bD. E. Yerien, S. Bonesi, A. Postigo, Org. Biomol. Chem. 2016, 14, 8398–8427.
- 5
- 5aC.-Y. Chen, H.-K. Jiang, B.-Q. Chen, M.-L. Liang, Acta Chim. Sin. 1966, 32, 18–25;
- 5bC. Hansch, A. Leo, R. W. Taft, Chem. Rev. 1991, 91, 165–195.
- 6
- 6aP. Bisel, L. Al-Momani, M. Müller, Org. Biomol. Chem. 2008, 6, 2655–2665;
- 6bA. K. Pandey, D. Naduthambi, K. M. Thomas, N. J. Zondlo, J. Am. Chem. Soc. 2013, 135, 4333–4363;
- 6cS. Decato, T. Bemis, E. Madsen, S. Mecozzi, Polym. Chem. 2014, 5, 6461–6471.
- 7For representative reviews, see:
- 7aJ.-X. Yu, V. D. Kodibagkar, W. Cui, R. P. Mason, Curr. Med. Chem. 2005, 12, 819–848;
- 7bE. N. G. Marsh, Y. Suzuki, ACS Chem. Biol. 2014, 9, 1242–1250;
- 7cT. M. Swager, Angew. Chem. Int. Ed. 2018, 57, 4248–4257; Angew. Chem. 2018, 130, 4325–4335;
- 7dZ. Xu, C. Liu, S. Zhao, S. Chen, Y. Zhao, Chem. Rev. 2019, 119, 195–230.
- 8For early synthesis of small molecules containing PFtB group using PFIB, see:
- 8aT. J. Brice, J. D. LaZerte, L. J. Hals, W. H. Pearlson, J. Am. Chem. Soc. 1953, 75, 2698–2702;
- 8bR. N. Haszeldine, J. M. Kidd, J. Chem. Soc. 1953, 3219–3225;
- 8cS. Andreades, J. Org. Chem. 1962, 27, 4163–4170.
- 9For leading references on the formal perfluoro-tert-butylation reactions using PFIB, see:
- 9aN. I. Delyagina, E. Y. Pervova, I. L. Knunyants, Russ Chem. Bull 1972, 21, 326–329;
10.1007/BF00857794 Google Scholar
- 9bB. L. Dyatkin, B. I. Martynov, L. G. Martynova, N. G. Kizim, S. R. Sterlin, Z. A. Stumbrevichute, L. A. Fedorov, J. Organomet. Chem. 1973, 57, 423–433;
- 9cS. L. Bell, R. D. Chambers, M. Y. Gribble, J. R. Maslakiewicz, J. Chem. Soc. Perkin Trans. 1 1973, 1716–1720;
- 9dY. V. Zeifman, L. T. Lantseva, I. L. Knunyants, Russ. Chem. Bull. 1978, 27, 2362–2366;
- 9eY. V. Zeifman, E. G. Ter-Gabrielyan, N. P. Gambaryan, I. L. Knunyants, Russ. Chem. Rev. 1984, 53, 256–273.
10.1070/RC1984v053n03ABEH003047 Google Scholar
- 10
- 10aI. L. Knunyants, S. T. Kocharyan, E. M. Rokhlin, Russ. Chem. Bull. 1966, 15, 1008–1012;
10.1007/BF00846056 Google Scholar
- 10bS. T. Kocharyan, E. M. Rokhlin, Y. A. Cheburkov, I. L. Knunyants, Russ. Chem. Bull. 1966, 15, 1813.
10.1007/BF00848744 Google Scholar
- 11E. P. Mochalina, B. L. Dyatkin, I. V. Galakhov, I. L. Knunyants, Dokl. Akad. Nauk SSSR 1966, 169, 1346–1349.
- 12T. Yajima, K. Yamaguchi, R. Hirokane, E. Nogami, J. Fluorine Chem. 2013, 150, 1–7.
- 13A. Kolomeitsev, A. Shtarev, K. Chabanenko, T. Savina, Y. Yagupolskii, A. Shtarev, K. Chabanenko, Y. Yagupolskii, M. Görg, J. Przyborowski, E. Lork, G.-V. Röschenthaler, Chem. Commun. 1998, 705–706.
- 14The reactions of C(CF3)3I with diazomethane and ethylene were described in Ref. [11], and only one example for perfluoro-tert-butylation was reported in Ref. [11] and Ref. [12].
- 15Q. Wang, C. Ni, M. Hu, Q. Xie, Q. Liu, S. Pan, J. Hu, Angew. Chem. Int. Ed. 2020, 59, 8507–8511; Angew. Chem. 2020, 132, 8585–8589.
- 16DBBF was first synthesized by Burton et al. in 1993, but no synthetic application of DBBF has been reported. See: P. A. Morken, P. C. Bachand, D. C. Swenson, D. J. Burton, J. Am. Chem. Soc. 1993, 115, 5430–5439.
- 17
- 17aB. E. Smart, W. J. Middleton, W. B. Farnham, J. Am. Chem. Soc. 1986, 108, 4905–4908;
- 17bN. I. Delyagina, S. M. Igumnov, V. F. Snegirev, I. L. Knunyants, Russ. Chem. Bull. 1981, 30, 1836–1840;
- 17cS. M. Igumnov, N. I. Delyagina, I. L. Knunyants, Russ. Chem. Bull. 1986, 35, 1191–1193.
- 18
- 18aW. B. Farnham, Chem. Rev. 1996, 96, 1633–1640;
- 18bD. A. Dixon, T. Fukunaga, B. E. Smart, J. Am. Chem. Soc. 1986, 108, 4027–4031;
- 18cW. B. Famham, B. E. Smart, W. J. Middleton, J. C. Calabrese, D. A. Dixon, J. Am. Chem. Soc. 1985, 107, 4565–4567;
- 18dW. B. Farnham, D. A. Dixon, J. C. Calabrese, J. Am. Chem. Soc. 1988, 110, 2607–2611.
- 19
- 19aJ. C. Jackson, J. T. Hammill, R. A. Mehl, J. Am. Chem. Soc. 2007, 129, 1160–1166;
- 19bN. B. Barhate, R. N. Barhate, P. Cekan, G. Drobny, S. T. Sigurdsson, Org. Lett. 2008, 10, 2745–2747;
- 19cB. C. Buer, B. J. Levin, E. N. G. Marsh, J. Pept. Sci. 2013, 19, 308–314;
- 19dC. M. Tressler, N. J. Zondlo, Org. Lett. 2016, 18, 6240–6243;
- 19eH. Meng, L. Wen, Z. Xu, Y. Li, J. Hao, Y. Zhao, Org. Lett. 2019, 21, 5206–5210.
- 20
- 20aJ. Clarke, M. Hatridge, M. Mössle, Annu. Rev. Biomed. Eng. 2007, 9, 389–413;
- 20bC. Liu, Y. Zhang, L. Qiu, H. Dong, H.-J. Krause, X. Xie, A. Offenhäusser, Supercond. Sci. Technol. 2012, 25, 075013.
- 21I. Tirotta, V. Dichiarante, C. Pigliacelli, G. Cavallo, G. Terraneo, F. B. Bombelli, P. Metrangolo, G. Resnati, Chem. Rev. 2015, 115, 1106–1129.
- 22Deposition Numbers 2042569 (for 3m) and 2042571 (for 3ap) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.