Virus-Mimicking Cell Membrane-Coated Nanoparticles for Cytosolic Delivery of mRNA
Joon Ho Park
Department of NanoEngineering, Chemical Engineering Program, Moores Cancer Center, University of California San Diego, La Jolla, CA, 92093 USA
Search for more papers by this authorAnimesh Mohapatra
Department of NanoEngineering, Chemical Engineering Program, Moores Cancer Center, University of California San Diego, La Jolla, CA, 92093 USA
Search for more papers by this authorJiarong Zhou
Department of NanoEngineering, Chemical Engineering Program, Moores Cancer Center, University of California San Diego, La Jolla, CA, 92093 USA
Search for more papers by this authorMaya Holay
Department of NanoEngineering, Chemical Engineering Program, Moores Cancer Center, University of California San Diego, La Jolla, CA, 92093 USA
Search for more papers by this authorNishta Krishnan
Department of NanoEngineering, Chemical Engineering Program, Moores Cancer Center, University of California San Diego, La Jolla, CA, 92093 USA
Search for more papers by this authorDr. Weiwei Gao
Department of NanoEngineering, Chemical Engineering Program, Moores Cancer Center, University of California San Diego, La Jolla, CA, 92093 USA
Search for more papers by this authorCorresponding Author
Dr. Ronnie H. Fang
Department of NanoEngineering, Chemical Engineering Program, Moores Cancer Center, University of California San Diego, La Jolla, CA, 92093 USA
Search for more papers by this authorCorresponding Author
Prof. Liangfang Zhang
Department of NanoEngineering, Chemical Engineering Program, Moores Cancer Center, University of California San Diego, La Jolla, CA, 92093 USA
Search for more papers by this authorJoon Ho Park
Department of NanoEngineering, Chemical Engineering Program, Moores Cancer Center, University of California San Diego, La Jolla, CA, 92093 USA
Search for more papers by this authorAnimesh Mohapatra
Department of NanoEngineering, Chemical Engineering Program, Moores Cancer Center, University of California San Diego, La Jolla, CA, 92093 USA
Search for more papers by this authorJiarong Zhou
Department of NanoEngineering, Chemical Engineering Program, Moores Cancer Center, University of California San Diego, La Jolla, CA, 92093 USA
Search for more papers by this authorMaya Holay
Department of NanoEngineering, Chemical Engineering Program, Moores Cancer Center, University of California San Diego, La Jolla, CA, 92093 USA
Search for more papers by this authorNishta Krishnan
Department of NanoEngineering, Chemical Engineering Program, Moores Cancer Center, University of California San Diego, La Jolla, CA, 92093 USA
Search for more papers by this authorDr. Weiwei Gao
Department of NanoEngineering, Chemical Engineering Program, Moores Cancer Center, University of California San Diego, La Jolla, CA, 92093 USA
Search for more papers by this authorCorresponding Author
Dr. Ronnie H. Fang
Department of NanoEngineering, Chemical Engineering Program, Moores Cancer Center, University of California San Diego, La Jolla, CA, 92093 USA
Search for more papers by this authorCorresponding Author
Prof. Liangfang Zhang
Department of NanoEngineering, Chemical Engineering Program, Moores Cancer Center, University of California San Diego, La Jolla, CA, 92093 USA
Search for more papers by this authorGraphical Abstract
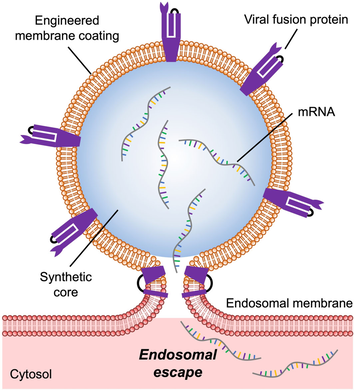
Cell membrane-coated nanoparticles are engineered to express a viral fusion protein, thus enabling them to exhibit improved endosomal escape properties. It is demonstrated that these virus-mimicking nanocarriers are able to deliver mRNA payloads to the cytosolic compartment after cellular uptake, enhancing expression of the encoded proteins both in vitro and in vivo.
Abstract
Effective endosomal escape after cellular uptake represents a major challenge in the field of nanodelivery, as the majority of drug payloads must localize to subcellular compartments other than the endosomes in order to exert activity. In nature, viruses can readily deliver their genetic material to the cytosol of host cells by triggering membrane fusion after endocytosis. For the influenza A virus, the hemagglutinin (HA) protein found on its surface fuses the viral envelope with the surrounding membrane at endosomal pH values. Biomimetic nanoparticles capable of endosomal escape were fabricated using a membrane coating derived from cells engineered to express HA on their surface. When evaluated in vitro, these virus-mimicking nanoparticles were able to deliver an mRNA payload to the cytosolic compartment of target cells, resulting in the successful expression of the encoded protein. When the mRNA-loaded nanoparticles were administered in vivo, protein expression levels were significantly increased in both local and systemic delivery scenarios. We therefore conclude that utilizing genetic engineering approaches to express viral fusion proteins on the surface of cell membrane-coated nanoparticles is a viable strategy for modulating the intracellular localization of encapsulated cargoes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202113671-sup-0001-misc_information.pdf518.5 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Y. Li, J. Wang, M. G. Wientjes, J. L. Au, Adv. Drug Delivery Rev. 2012, 64, 29.
- 2L. Mocan, C. Matea, F. A. Tabaran, O. Mosteanu, T. Pop, T. Mocan, C. Iancu, Int. J. Nanomed. 2015, 10, 5435.
- 3D. H. Dam, J. H. Lee, P. N. Sisco, D. T. Co, M. Zhang, M. R. Wasielewski, T. W. Odom, ACS Nano 2012, 6, 3318.
- 4A. Fu, R. Tang, J. Hardie, M. E. Farkas, V. M. Rotello, Bioconjugate Chem. 2014, 25, 1602.
- 5L. D. Field, J. B. Delehanty, Y. Chen, I. L. Medintz, Acc. Chem. Res. 2015, 48, 1380.
- 6B. He, P. Lin, Z. Jia, W. Du, W. Qu, L. Yuan, W. Dai, H. Zhang, X. Wang, J. Wang, X. Zhang, Q. Zhang, Biomaterials 2013, 34, 6082.
- 7S. Biswas, V. P. Torchilin, Adv. Drug Delivery Rev. 2014, 66, 26.
- 8J. Huotari, A. Helenius, EMBO J. 2011, 30, 3481.
- 9C. C. Scott, F. Vacca, J. Gruenberg, Semin. Cell Dev. Biol. 2014, 31, 2.
- 10C. Zhang, T. An, D. Wang, G. Wan, M. Zhang, H. Wang, S. Zhang, R. Li, X. Yang, Y. Wang, J. Controlled Release 2016, 226, 193.
- 11Z. Zhou, A. Badkas, M. Stevenson, J. Y. Lee, Y. K. Leung, Int. J. Pharm. 2015, 487, 81.
- 12S. A. Smith, L. I. Selby, A. P. R. Johnston, G. K. Such, Bioconjugate Chem. 2019, 30, 263.
- 13A. J. Convertine, D. S. Benoit, C. L. Duvall, A. S. Hoffman, P. S. Stayton, J. Controlled Release 2009, 133, 221.
- 14K. K. Tran, X. Zhan, H. Shen, Adv. Healthcare Mater. 2014, 3, 690.
- 15X. A. Wu, C. H. Choi, C. Zhang, L. Hao, C. A. Mirkin, J. Am. Chem. Soc. 2014, 136, 7726.
- 16E. Hinde, K. Thammasiraphop, H. T. Duong, J. Yeow, B. Karagoz, C. Boyer, J. J. Gooding, K. Gaus, Nat. Nanotechnol. 2017, 12, 81.
- 17S. P. Mukherjee, H. J. Byrne, Nanomedicine 2013, 9, 202.
- 18H. Lv, S. Zhang, B. Wang, S. Cui, J. Yan, J. Controlled Release 2006, 114, 100.
- 19J. Staring, M. Raaben, T. R. Brummelkamp, J. Cell Sci. 2018, 131, jcs216259.
- 20B. S. Hamilton, G. R. Whittaker, S. Daniel, Viruses 2012, 4, 1144.
- 21C. J. Russell, M. Hu, F. A. Okda, Trends Microbiol. 2018, 26, 841.
- 22S. Boonstra, J. S. Blijleven, W. H. Roos, P. R. Onck, E. van der Giessen, A. M. van Oijen, Annu. Rev. Biophys. 2018, 47, 153.
- 23S. G. Lazarowitz, P. W. Choppin, Virology 1975, 68, 440.
- 24S. A. Tatulian, L. K. Tamm, Biochemistry 2000, 39, 496.
- 25S. Thoennes, Z. N. Li, B. J. Lee, W. A. Langley, J. J. Skehel, R. J. Russell, D. A. Steinhauer, Virology 2008, 370, 403.
- 26R. Xu, I. A. Wilson, J. Virol. 2011, 85, 5172.
- 27C. S. Kim, R. F. Epand, E. Leikina, R. M. Epand, L. V. Chernomordik, J. Biol. Chem. 2011, 286, 13226.
- 28X. Han, J. H. Bushweller, D. S. Cafiso, L. K. Tamm, Nat. Struct. Biol. 2001, 8, 715.
- 29M. N. Matrosovich, T. Y. Matrosovich, T. Gray, N. A. Roberts, H. D. Klenk, Proc. Natl. Acad. Sci. USA 2004, 101, 4620.
- 30A. Ibricevic, A. Pekosz, M. J. Walter, C. Newby, J. T. Battaile, E. G. Brown, M. J. Holtzman, S. L. Brody, J. Virol. 2006, 80, 7469.
- 31A. C. Monsalvo, J. P. Batalle, M. F. Lopez, J. C. Krause, J. Klemenc, J. Z. Hernandez, B. Maskin, J. Bugna, C. Rubinstein, L. Aguilar, L. Dalurzo, R. Libster, V. Savy, E. Baumeister, L. Aguilar, G. Cabral, J. Font, L. Solari, K. P. Weller, J. Johnson, M. Echavarria, K. M. Edwards, J. D. Chappell, J. E. Crowe, Jr., J. V. Williams, G. A. Melendi, F. P. Polack, Nat. Med. 2011, 17, 195.
- 32R. H. Fang, A. V. Kroll, W. Gao, L. Zhang, Adv. Mater. 2018, 30, 1706759.
- 33R. H. Fang, Y. Jiang, J. C. Fang, L. Zhang, Biomaterials 2017, 128, 69.
- 34C. M. Hu, L. Zhang, S. Aryal, C. Cheung, R. H. Fang, L. Zhang, Proc. Natl. Acad. Sci. USA 2011, 108, 10980.
- 35R. H. Fang, C. M. Hu, B. T. Luk, W. Gao, J. A. Copp, Y. Tai, D. E. O'Connor, L. Zhang, Nano Lett. 2014, 14, 2181.
- 36S. Wang, Y. Duan, Q. Zhang, A. Komarla, H. Gong, W. Gao, L. Zhang, Small Struct. 2020, 1, 2000018.
- 37Y. Jiang, N. Krishnan, J. Zhou, S. Chekuri, X. Wei, A. V. Kroll, C. L. Yu, Y. Duan, W. Gao, R. H. Fang, L. Zhang, Adv. Mater. 2020, 32, 2001808.
- 38J. H. Park, Y. Jiang, J. Zhou, H. Gong, A. Mohapatra, J. Heo, W. Gao, R. H. Fang, L. Zhang, Sci. Adv. 2021, 7, eabf7820.
- 39R. Verbeke, I. Lentacker, S. C. De Smedt, H. Dewitte, J. Controlled Release 2021, 333, 511.
- 40S. E. Galloway, M. L. Reed, C. J. Russell, D. A. Steinhauer, PLoS Pathog. 2013, 9, e1003151.
- 41B. Su, S. Wurtzer, M. A. Rameix-Welti, D. Dwyer, S. van der Werf, N. Naffakh, F. Clavel, B. Labrosse, PLoS One 2009, 4, e8495.
- 42M. J. Ma, Y. Yang, L. Q. Fang, Trends Microbiol. 2019, 27, 93.
- 43W. W. Chang, C. Y. Yu, T. W. Lin, P. H. Wang, Y. C. Tsai, Biochem. Biophys. Res. Commun. 2006, 341, 614.
- 44A. V. Kroll, R. H. Fang, Y. Jiang, J. Zhou, X. Wei, C. L. Yu, J. Gao, B. T. Luk, D. Dehaini, W. Gao, L. Zhang, Adv. Mater. 2017, 29, 1703969.
- 45J. A. Copp, R. H. Fang, B. T. Luk, C. M. Hu, W. Gao, K. Zhang, L. Zhang, Proc. Natl. Acad. Sci. USA 2014, 111, 13481.
- 46M. A. Islam, Y. Xu, W. Tao, J. M. Ubellacker, M. Lim, D. Aum, G. Y. Lee, K. Zhou, H. Zope, M. Yu, W. Cao, J. T. Oswald, M. Dinarvand, M. Mahmoudi, R. Langer, P. W. Kantoff, O. C. Farokhzad, B. R. Zetter, J. Shi, Nat. Biomed. Eng. 2018, 2, 850.
- 47X. Xu, K. Xie, X. Q. Zhang, E. M. Pridgen, G. Y. Park, D. S. Cui, J. Shi, J. Wu, P. W. Kantoff, S. J. Lippard, R. Langer, G. C. Walker, O. C. Farokhzad, Proc. Natl. Acad. Sci. USA 2013, 110, 18638.
- 48J. Martinez-Fabregas, A. Prescott, S. van Kasteren, D. L. Pedrioli, I. McLean, A. Moles, T. Reinheckel, V. Poli, C. Watts, Nat. Commun. 2018, 9, 5343.
- 49J. Yoon, S. H. Bang, J. S. Park, S. T. Chang, Y. H. Kim, J. Min, Appl. Biochem. Biotechnol. 2011, 163, 1002.
- 50K. Blanchfield, R. P. Kamal, W. P. Tzeng, N. Music, J. R. Wilson, J. Stevens, A. S. Lipatov, J. M. Katz, I. A. York, Influenza Other Respir. Viruses 2014, 8, 628.