Brønsted Acid Catalyzed Dearomatization by Intramolecular Hydroalkoxylation/Claisen Rearrangement: Diastereo- and Enantioselective Synthesis of Spirolactams
Peng-Fei Chen
State Key Laboratory of Physical Chemistry of Solid Surfaces, Key Laboratory of Chemical Biology of Fujian Province, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
These authors contributed equally to this work.
Search for more papers by this authorBo Zhou
State Key Laboratory of Physical Chemistry of Solid Surfaces, Key Laboratory of Chemical Biology of Fujian Province, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
These authors contributed equally to this work.
Search for more papers by this authorPeng Wu
State Key Laboratory of Physical Chemistry of Solid Surfaces, Key Laboratory of Chemical Biology of Fujian Province, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorProf. Dr. Binju Wang
State Key Laboratory of Physical Chemistry of Solid Surfaces, Key Laboratory of Chemical Biology of Fujian Province, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Long-Wu Ye
State Key Laboratory of Physical Chemistry of Solid Surfaces, Key Laboratory of Chemical Biology of Fujian Province, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
State Key Laboratory of Organometallic Chemistry, Shanghai, Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032 China
State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorPeng-Fei Chen
State Key Laboratory of Physical Chemistry of Solid Surfaces, Key Laboratory of Chemical Biology of Fujian Province, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
These authors contributed equally to this work.
Search for more papers by this authorBo Zhou
State Key Laboratory of Physical Chemistry of Solid Surfaces, Key Laboratory of Chemical Biology of Fujian Province, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
These authors contributed equally to this work.
Search for more papers by this authorPeng Wu
State Key Laboratory of Physical Chemistry of Solid Surfaces, Key Laboratory of Chemical Biology of Fujian Province, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorProf. Dr. Binju Wang
State Key Laboratory of Physical Chemistry of Solid Surfaces, Key Laboratory of Chemical Biology of Fujian Province, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Long-Wu Ye
State Key Laboratory of Physical Chemistry of Solid Surfaces, Key Laboratory of Chemical Biology of Fujian Province, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
State Key Laboratory of Organometallic Chemistry, Shanghai, Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032 China
State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorDedicated to the 100th anniversary of Xiamen University
Graphical Abstract
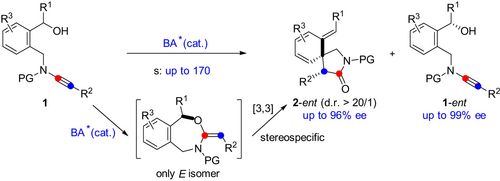
A Brønsted acid catalyzed intramolecular hydroalkoxylation/Claisen rearrangement is disclosed that involves an unexpected dearomatization of nonactivated arenes and heteroaromatic compounds and allows the practical and atom-economic synthesis of various valuable spirolactams. Moreover, the asymmetric version of this tandem cyclization is also achieved via kinetic resolution by chiral phosphoric acid catalysis.
Abstract
Described herein is a novel Brønsted acid catalyzed intramolecular hydroalkoxylation/Claisen rearrangement, allowing the practical and atom-economic synthesis of a range of valuable spirolactams from readily available ynamides in generally good to excellent yields with excellent diastereoselectivities and broad substrate scope. Importantly, an unexpected dearomatization of nonactivated arenes and heteroaromatic compounds is involved in this tandem sequence. Moreover, an asymmetric version of this tandem cyclization was also achieved by efficient kinetic resolution by chiral phosphoric acid catalysis. In addition, the [3,3]-rearrangement is shown to be kinetically preferred over the related [1,3]-rearrangement by theoretical calculations.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202113464-sup-0001-cif.zip478.5 KB | Supporting Information |
| anie202113464-sup-0001-misc_information.pdf10.6 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. J. Huck, D. Sarlah, Chem 2020, 6, 1589;
- 1bM. P. Wiesenfeldt, Z. Nairoukh, T. Dalton, F. Glorius, Angew. Chem. Int. Ed. 2019, 58, 10460; Angew. Chem. 2019, 131, 10570;
- 1cB. K. Liebov, W. D. Harman, Chem. Rev. 2017, 117, 13721;
- 1dR. Remy, C. G. Bochet, Chem. Rev. 2016, 116, 9816;
- 1eD.-S. Wang, Q.-A. Chen, S.-M. Lu, Y.-G. Zhou, Chem. Rev. 2012, 112, 2557;
- 1fS. P. Roche, J. A. Porco, Jr., Angew. Chem. Int. Ed. 2011, 50, 4068; Angew. Chem. 2011, 123, 4154;
- 1gF. L. Ortiz, M. J. Iglesias, I. Fernández, C. M. A. Sánchez, G. R. Gómez, Chem. Rev. 2007, 107, 1580;
- 1hA. R. Pape, K. P. Kaliappan, E. P. Kündig, Chem. Rev. 2000, 100, 2917.
- 2W. C. Wertjes, E. H. Southgate, D. Sarlah, Chem. Soc. Rev. 2018, 47, 7996.
- 3
- 3aJ. P. Cole, D.-F. Chen, M. Kudisch, R. M. Pearson, C.-H. Lim, G. M. Miyake, J. Am. Chem. Soc. 2020, 142, 13573;
- 3bA. Chatterjee, B. König, Angew. Chem. Int. Ed. 2019, 58, 14289; Angew. Chem. 2019, 131, 14427;
- 3cP. Lei, Y. Ding, X. Zhang, A. Adijiang, H. Li, Y. Ling, J. An, Org. Lett. 2018, 20, 3439;
- 3dM. M. Heravi, M. V. Fard, Z. Faghihi, Curr. Org. Chem. 2015, 19, 1491;
- 3eH. E. Zimmerman, Acc. Chem. Res. 2012, 45, 164;
- 3fT. J. Donohoe, R. Garg, C. A. Stevenson, Tetrahedron: Asymmetry 1996, 7, 317;
- 3gA. J. Birch, J. Chem. Soc. 1944, 430.
- 4
- 4aK. S. Weddle, J. D. Aiken III, R. G. Finke, J. Am. Chem. Soc. 1998, 120, 5653;
- 4bS. D. Lin, M. A. Vannice, J. Catal. 1993, 143, 539;
- 4cJ. S. Lee, M. H. Yeom, K. Y. Park, I.-S. Nam, J. S. Chung, Y. G. Kim, S. H. Moon, J. Catal. 1991, 128, 126.
- 5
- 5aA. S. Alshreimi, G. Zhang, T. W. Reidl, R. L. Peña, N.-G. Koto, S. M. Islam, D. J. Wink, L. L. Anderson, Angew. Chem. Int. Ed. 2020, 59, 15244; Angew. Chem. 2020, 132, 15356;
- 5bH. Zheng, Y. Wang, C. Xu, X. Xu, L. Lin, X. Liu, X. Feng, Nat. Commun. 2018, 9, 1968;
- 5cM. T. Peruzzi, S. J. Lee, M. R. Gagné, Org. Lett. 2017, 19, 6256;
- 5dJ. An, A. Parodi, M. Monari, M. C. Reis, C. S. Lopez, M. Bandini, Chem. Eur. J. 2017, 23, 17473;
- 5eT. Cao, E. C. Linton, J. Deitch, S. Berritt, M. C. Kozlowski, J. Org. Chem. 2012, 77, 11034;
- 5fT. Cao, J. Deitch, E. C. Linton, M. C. Kozlowski, Angew. Chem. Int. Ed. 2012, 51, 2448; Angew. Chem. 2012, 124, 2498;
- 5gE. C. Linton, M. C. Kozlowski, J. Am. Chem. Soc. 2008, 130, 16162;
- 5hfor a recent gold-catalyzed thio-Claisen reaction for the formation of a quaternary all C-center, see: H. Kim, J. Jang, S. Shin, J. Am. Chem. Soc. 2020, 142, 20788.
- 6
- 6aS. Huang, L. Kötzner, C. K. De, B. List, J. Am. Chem. Soc. 2015, 137, 3446;
- 6bL. Kötzner, M. Leutzsch, S. Sievers, S. Patil, H. Waldmann, Y. Zheng, W. Thiel, B. List, Angew. Chem. Int. Ed. 2016, 55, 7693; Angew. Chem. 2016, 128, 7824.
- 7
- 7aB. Zhou, Y.-Q. Zhang, K. Zhang, M.-Y. Yang, Y.-B. Chen, Y. Li, Q. Peng, S.-F. Zhu, Q.-L. Zhou, L.-W. Ye, Nat. Commun. 2019, 10, 3234;
- 7bL. Li, X.-Q. Zhu, Y.-Q. Zhang, H.-Z. Bu, P. Yuan, J. Chen, J. Su, X. Deng, L.-W. Ye, Chem. Sci. 2019, 10, 3123;
- 7cB. Zhou, L. Li, X.-Q. Zhu, J.-Z. Yan, Y.-L. Guo, L.-W. Ye, Angew. Chem. Int. Ed. 2017, 56, 4015; Angew. Chem. 2017, 129, 4073.
- 8For recent reviews on ynamide reactivity, see:
- 8aY.-C. Hu, Y. Zhao, B. Wan, Q.-A. Chen, Chem. Soc. Rev. 2021, 50, 2582;
- 8bY.-B. Chen, P.-C. Qian, L.-W. Ye, Chem. Soc. Rev. 2020, 49, 8897;
- 8cC. C. Lynch, A. Sripada, C. Wolf, Chem. Soc. Rev. 2020, 49, 8543;
- 8dJ. Luo, G.-S. Chen, S.-J. Chen, J.-S. Yu, Z.-D. Li, Y.-L. Liu, ACS Catal. 2020, 10, 13978;
- 8eB. Zhou, T.-D. Tan, X.-Q. Zhu, M. Shang, L.-W. Ye, ACS Catal. 2019, 9, 6393;
- 8fG. Evano, C. Theunissen, M. Lecomte, Aldrichimica Acta 2015, 48, 59;
- 8gX.-N. Wang, H.-S. Yeom, L.-C. Fang, S. He, Z.-X. Ma, B. L. Kedrowski, R. P. Hsung, Acc. Chem. Res. 2014, 47, 560;
- 8hK. A. DeKorver, H. Li, A. G. Lohse, R. Hayashi, Z. Lu, Y. Zhang, R. P. Hsung, Chem. Rev. 2010, 110, 5064;
- 8iG. Evano, A. Coste, K. Jouvin, Angew. Chem. Int. Ed. 2010, 49, 2840; Angew. Chem. 2010, 122, 2902.
- 9For a review on catalytic tandem reactions of ynamides by our group, see:
- 9aF.-L. Hong, L.-W. Ye, Acc. Chem. Res. 2020, 53, 2003; for recent selected examples from our group, see:
- 9bY.-Q. Zhang, Y.-P. Zhang, Y.-X. Zheng, Z.-Y. Li, L.-W. Ye, Cell Rep. Phys. Sci. 2021, 2, 100448;
- 9cX.-Q. Zhu, P. Hong, Y.-X. Zheng, Y.-Y. Zhen, F.-L. Hong, X. Lu, L.-W. Ye, Chem. Sci. 2021, 12, 9466;
- 9dF.-L. Hong, Y.-B. Chen, S.-H. Ye, G.-Y. Zhu, X.-Q. Zhu, X. Lu, R.-S. Liu, L.-W. Ye, J. Am. Chem. Soc. 2020, 142, 7618;
- 9eZ.-S. Wang, Y.-B. Chen, H.-W. Zhang, Z. Sun, C. Zhu, L.-W. Ye, J. Am. Chem. Soc. 2020, 142, 3636;
- 9fX. Liu, Z.-S. Wang, T.-Y. Zhai, C. Luo, Y.-P. Zhang, Y.-B. Chen, C. Deng, R.-S. Liu, L.-W. Ye, Angew. Chem. Int. Ed. 2020, 59, 17984; Angew. Chem. 2020, 132, 18140;
- 9gF.-L. Hong, Z.-S. Wang, D.-D. Wei, T.-Y. Zhai, G.-C. Deng, X. Lu, R.-S. Liu, L.-W. Ye, J. Am. Chem. Soc. 2019, 141, 16961;
- 9hY. Xu, Q. Sun, T.-D. Tan, M.-Y. Yang, P. Yuan, S.-Q. Wu, X. Lu, X. Hong, L.-W. Ye, Angew. Chem. Int. Ed. 2019, 58, 16252; Angew. Chem. 2019, 131, 16398.
- 10For recent reviews on kinetic resolution via chiral phosphoric acid catalysis, see:
- 10aW. Liu, X. Yang, Asian J. Org. Chem. 2021, 10, 692;
- 10bK. S. Petersen, Asian J. Org. Chem. 2016, 5, 308;
- 10cR. Gurubrahamam, Y.-S. Cheng, W.-Y. Huang, K. Chen, ChemCatChem 2016, 8, 86; for selected examples, see:
- 10dM. Tang, H. Gu, S. He, S. Rajkumar, X. Yang, Angew. Chem. Int. Ed. 2021, 60, 21334; Angew. Chem. 2021, 133, 21504;
- 10eY. Chen, C. Zhu, Z. Guo, W. Liu, X. Yang, Angew. Chem. Int. Ed. 2021, 60, 5268; Angew. Chem. 2021, 133, 5328;
- 10fW. Liu, Q. Jiang, X. Yang, Angew. Chem. Int. Ed. 2020, 59, 23598; Angew. Chem. 2020, 132, 23804;
- 10gS. Rajkumar, M. Tang, X. Yang, Angew. Chem. Int. Ed. 2020, 59, 2333; Angew. Chem. 2020, 132, 2353;
- 10hS. Rajkumar, S. He, X. Yang, Angew. Chem. Int. Ed. 2019, 58, 10315; Angew. Chem. 2019, 131, 10421;
- 10iH. Yang, W.-H. Zheng, Org. Lett. 2019, 21, 5197;
- 10jI. Čorić, J. H. Kim, T. Vlaar, M. Patil, W. Thiel, B. List, Angew. Chem. Int. Ed. 2013, 52, 3490; Angew. Chem. 2013, 125, 3574;
- 10kI. Čorić, S. Müller, B. List, J. Am. Chem. Soc. 2010, 132, 17370.
- 11The Brønsted acid catalyzed cascade cyclization of ynamides 3 a–3 d failed to afford the desired products.

- 12For details, see the Supporting Information (SI).
- 13Deposition Numbers 2077182 (2ac), 2077183 (for (+)-2j), 2077184 (for 4), 2077185 (for 5), and 2110706 (for 2w′) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 14For recent reviews on CADA, see:
- 14aC. Zheng, S.-L. You, ACS Cent. Sci. 2021, 7, 432;
- 14bZ.-L. Xia, Q.-F. Xu-Xu, C. Zheng, S.-L. You, Chem. Soc. Rev. 2020, 49, 286;
- 14cC. Zheng, S.-L. You, Nat. Prod. Rep. 2019, 36, 1589;
- 14dW.-T. Wu, L. Zhang, S.-L. You, Chem. Soc. Rev. 2016, 45, 1570;
- 14eC. Zheng, S.-L. You, Chem 2016, 1, 830.
- 15For CADA of electron-deficient alkynes by Brønsted acids, see:
- 15aJ. Yang, Z. Wang, Z. He, G. Li, L. Hong, W. Sun, R. Wang, Angew. Chem. Int. Ed. 2020, 59, 642; Angew. Chem. 2020, 132, 652;
- 15bX. Liu, J. Zhang, L. Bai, L. Wang, D. Yang, R. Wang, Chem. Sci. 2020, 11, 671;
- 15cD. Qian, L. Wu, Z. Lin, J. Sun, Nat. Commun. 2017, 8, 567.
- 16This spiro γ-lactam skeleton can be found in a variety of bioactive compounds, see recent examples:
- 16aR. K. Gamidi, Å. C. Rasmuson, Cryst. Growth Des. 2020, 20, 5745;
- 16bA. V. Baranovskii, M. B. Golubeva, Chem. Nat. Compd. 2016, 52, 856;
- 16cF. Felluga, F. Ghelfi, G. Pitacco, F. Roncaglia, E. Valentin, C. D. Venneri, Tetrahedron: Asymmetry 2010, 21, 2183;
- 16dC. Ovens, N. G. Martin, D. J. Procter, Org. Lett. 2008, 10, 1441;
- 16eB. Shaheen Siddiqui, S. B. Usmani, S. T. Ali, S. Begum, G. H. Rizwani, Heterocycles 2003, 60, 909;
10.3987/COM-02-9652 Google Scholar
- 16fW. M. Kazmierski, E. Furfine, A. Spaltenstein, L. L. Wright, Bioorg. Med. Chem. Lett. 2002, 12, 3431.