A Face-to-Face Dimer of Au3 Superatoms Supported by Interlocked Tridentate Scaffolds Formed in Au18S2(SR)12
Taro Shigeta
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 1130033 Japan
Search for more papers by this authorDr. Shinjiro Takano
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 1130033 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Tatsuya Tsukuda
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 1130033 Japan
Elements Strategy Initiative for Catalysts and Batteries (ESICB), Kyoto University, 1-30 Goryo-Ohara, Nishikyo-ku, Kyoto, 6158245 Japan
Search for more papers by this authorTaro Shigeta
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 1130033 Japan
Search for more papers by this authorDr. Shinjiro Takano
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 1130033 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Tatsuya Tsukuda
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 1130033 Japan
Elements Strategy Initiative for Catalysts and Batteries (ESICB), Kyoto University, 1-30 Goryo-Ohara, Nishikyo-ku, Kyoto, 6158245 Japan
Search for more papers by this authorGraphical Abstract
Abstract
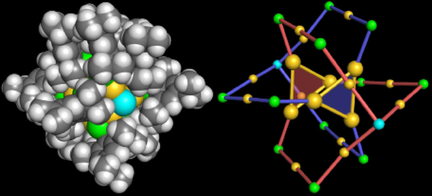
A new sulfur-containing gold cluster, Au18S2(STipb)12, was serendipitously obtained using the bulky thiol, 2,4,6-triisopropylbenzyl mercaptan (TipbSH), as protecting ligands. Single-crystal X-ray diffraction analysis revealed that Au18S2(STipb)12 has a deformed octahedral Au6 core clutched by two tridentate S[Au2(STipb)2]3 units in an interlocked manner. Based on density functional theory calculations, we propose that the Au6 core with two electrons is better viewed as a face-to-face dimer of Au3(1e) superatoms rather than an electronically closed Au6(2e) superatom. In situ formation of the sulfide anions (S2−) via C-S bond breakage is ascribed to the steric repulsion between the TipbS ligands.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202113275-sup-0001-misc_information.pdf1.5 MB | Supporting Information |
| anie202113275-sup-0001-Movie_S1.mp42.3 MB | Supporting Information |
| anie202113275-sup-0001-Movie_S2.mp42.2 MB | Supporting Information |
| anie202113275-sup-0001-Movie_S3.mp41.6 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. Jin, C. Zhen, M. Zhou, Y. Chen, Chem. Rev. 2016, 116, 10346–10413.
- 2I. Chakraborty, T. Pradeep, Chem. Rev. 2017, 117, 8208–8271.
- 3H. Hirai, S. Ito, S. Takano, K. Koyasu, T. Tsukuda, Chem. Sci. 2020, 11, 12233–12248.
- 4M. Walter, J. Akola, O. Lopez-Acevedo, P. D. Jadzinsky, G. Calero, C. J. Ackerson, R. L. Whetten, H. Grönbeck, H. Häkkinen, Proc. Natl. Acad. Sci. USA 2008, 105, 9157–9162.
- 5S. Takano, T. Tsukuda, J. Am. Chem. Soc. 2021, 143, 1683–1698.
- 6T. Omoda, S. Takano, T. Tsukuda, Small 2021, 17, 2001439.
- 7L. Cheng, C. Ren, X. Zhang, J. Yang, Nanoscale 2013, 5, 1475–1478.
- 8J. Nishigaki, K. Koyasu, T. Tsukuda, Chem. Rec. 2014, 14, 897–909.
- 9T. Dainese, S. Antonello, S. Bogialli, W. Fei, A. Venzo, F. Maran, ACS Nano 2018, 12, 7057–7066.
- 10E. Ito, S. Takano, T. Nakamura, T. Tsukuda, Angew. Chem. Int. Ed. 2021, 60, 645–649; Angew. Chem. 2021, 133, 655–659.
- 11M. Brust, M. Walker, D. Bethell, D. J. Schiffrin, R. Whyman, J. Chem. Soc. Chem. Commun. 1994, 801–802.
- 12Y. Pei, P. Wang, Z. Ma, L. Xiong, Acc. Chem. Res. 2019, 52, 23–33.
- 13H. Häkkinen, Nat. Chem. 2012, 4, 443–455.
- 14T. Higaki, C. Zeng, Y. Chen, E. Hussain, R. Jin, CrystEngComm 2016, 18, 6979–6986.
- 15S. Hossain, Y. Imai, D. Suzuki, W. Choi, Z. Chen, T. Suzuki, M. Yoshioka, T. Kawawaki, D. Lee, Y. Negishi, Nanoscale 2019, 11, 22089–22098.
- 16T. Omoda, S. Takano, S. Yamazoe, K. Koyasu, Y. Negishi, T. Tsukuda, J. Phys. Chem. C 2018, 122, 13199–13204.
- 17D. Crasto, S. Malola, G. Brosofsky, A. Dass, H. Häkkinen, J. Am. Chem. Soc. 2014, 136, 5000–5005.
- 18C. Liu, T. Li, G. Li, K. Nobusada, C. Zeng, G. Pang, N. L. Rosi, R. Jin, Angew. Chem. Int. Ed. 2015, 54, 9826–9829; Angew. Chem. 2015, 127, 9964–9967.
- 19T. C. Jones, L. Sementa, M. Stener, K. J. Gagnon, V. D. Thanthirige, G. Ramakrishna, A. Fortunelli, A. Dass, J. Phys. Chem. C 2017, 121, 10865–10869.
- 20T. Higaki, C. Liu, M. Zhou, T.-Y. Luo, N. L. Rosi, R. Jin, J. Am. Chem. Soc. 2017, 139, 9994–10001.
- 21J. Nishigaki, R. Tsunoyama, H. Tsunoyama, N. Ichikuni, S. Yamazoe, Y. Negishi, M. Ito, T. Matsuo, K. Tamao, T. Tsukuda, J. Am. Chem. Soc. 2012, 134, 14295–14297.
- 22J. Nishigaki, S. Yamazoe, S. Kohara, A. Fujiwara, W. Kurashige, Y. Negishi, T. Tsukuda, Chem. Commun. 2014, 50, 839–841.
- 23T. Omoda, S. Takano, T. Tsukuda, Chem. Lett. 2019, 48, 885–887.
- 24Deposition Number 2067702 contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 25J. B. Tracy, M. C. Crowe, J. F. Parker, O. Hampe, C. A. Fields-Zinna, A. Dass, R. W. Murray, J. Am. Chem. Soc. 2007, 129, 16209–16215.
- 26M. Fujita, N. Fujita, K. Ogura, K. Yamaguchi, Nature 1999, 400, 52–55.
- 27M. R. Wiseman, P. A. Marsh, P. T. Bishop, B. J. Brisdon, M. F. Mahon, J. Am. Chem. Soc. 2000, 122, 12598–12599.
- 28S. S.-Y. Chui, R. Chen, C.-M. Che, Angew. Chem. Int. Ed. 2006, 45, 1621–1624; Angew. Chem. 2006, 118, 1651–1654.
- 29A. Das, T. Li, G. Li, K. Nobusada, C. Zeng, N. L. Rosi, R. Jin, Nanoscale 2014, 6, 6458–6462.
- 30F. Demartin, M. Manassero, L. Naldini, R. Ruggeri, M. Sansoni, J. Chem. Soc. Chem. Commun. 1981, 222–223.
- 31J. W. A. van der Velden, J. J. Bour, R. Pet, W. P. Bosman, J. H. Noordik, Inorg. Chem. 1983, 22, 3112–3115.
- 32E. Zeller, H. Beruda, H. Schmidbaur, Inorg. Chem. 1993, 32, 3203–3204.
- 33Y. Yang, P. R. Sharp, J. Am. Chem. Soc. 1994, 116, 6983–6984.
- 34M. J. Calhorda, O. Crespo, M. C. Gimeno, P. G. Jones, A. Laguna, J. M. López-de-Luzuriaga, J. L. Perez, M. A. Ramón, L. F. Veiros, Inorg. Chem. 2000, 39, 4280–4285.
- 35D. Zhang, J. Dou, D. Li, D. Wang, J. Coord. Chem. 2007, 60, 825–831.
- 36A. Das, C. Liu, H. Y. Byun, K. Nobusada, S. Zhao, N. Rosi, R. Jin, Angew. Chem. Int. Ed. 2015, 54, 3140–3144; Angew. Chem. 2015, 127, 3183–3187.
- 37S. Chen, S. Wang, J. Zhong, Y. Song, J. Zhang, H. Sheng, Y. Pei, M. Zhu, Angew. Chem. Int. Ed. 2015, 54, 3145–3149; Angew. Chem. 2015, 127, 3188–3192.
- 38Y. Negishi, K. Nobusada, T. Tsukuda, J. Am. Chem. Soc. 2005, 127, 5261–5270.
- 39Q. Yao, Y. Yu, X. Yuan, Y. Yu, J. Xie, J. Y. Lee, Small 2013, 9, 2696–2701.
- 40R. Itteboina, U. D. Madhuri, P. Ghosal, M. Kannan, T. K. Sau, T. Tsukuda, S. Bhardwaj, J. Phys. Chem. A 2018, 122, 1228–1234.