An Iridium Catalytic System Compatible with Inorganic and Organic Nitrogen Sources for Dual Asymmetric Reductive Amination Reactions
Zhaofeng Gao
Department of Chemistry, Shaanxi Key Laboratory of Natural Products & Chemical Biology, Northwest A&F University, 22 Xinong Road, Yangling, Shaanxi, 712100 P. R. China
Search for more papers by this authorJingwen Liu
Department of Chemistry, Shaanxi Key Laboratory of Natural Products & Chemical Biology, Northwest A&F University, 22 Xinong Road, Yangling, Shaanxi, 712100 P. R. China
Search for more papers by this authorHaizhou Huang
Department of Chemistry, Shaanxi Key Laboratory of Natural Products & Chemical Biology, Northwest A&F University, 22 Xinong Road, Yangling, Shaanxi, 712100 P. R. China
Search for more papers by this authorCorresponding Author
Huiling Geng
Department of Chemistry, Shaanxi Key Laboratory of Natural Products & Chemical Biology, Northwest A&F University, 22 Xinong Road, Yangling, Shaanxi, 712100 P. R. China
Search for more papers by this authorCorresponding Author
Mingxin Chang
Department of Chemistry, Shaanxi Key Laboratory of Natural Products & Chemical Biology, Northwest A&F University, 22 Xinong Road, Yangling, Shaanxi, 712100 P. R. China
Search for more papers by this authorZhaofeng Gao
Department of Chemistry, Shaanxi Key Laboratory of Natural Products & Chemical Biology, Northwest A&F University, 22 Xinong Road, Yangling, Shaanxi, 712100 P. R. China
Search for more papers by this authorJingwen Liu
Department of Chemistry, Shaanxi Key Laboratory of Natural Products & Chemical Biology, Northwest A&F University, 22 Xinong Road, Yangling, Shaanxi, 712100 P. R. China
Search for more papers by this authorHaizhou Huang
Department of Chemistry, Shaanxi Key Laboratory of Natural Products & Chemical Biology, Northwest A&F University, 22 Xinong Road, Yangling, Shaanxi, 712100 P. R. China
Search for more papers by this authorCorresponding Author
Huiling Geng
Department of Chemistry, Shaanxi Key Laboratory of Natural Products & Chemical Biology, Northwest A&F University, 22 Xinong Road, Yangling, Shaanxi, 712100 P. R. China
Search for more papers by this authorCorresponding Author
Mingxin Chang
Department of Chemistry, Shaanxi Key Laboratory of Natural Products & Chemical Biology, Northwest A&F University, 22 Xinong Road, Yangling, Shaanxi, 712100 P. R. China
Search for more papers by this authorGraphical Abstract
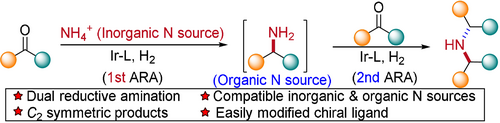
Two successive asymmetric reductive aminations (ARAs) have been merged into one reaction system to generate enantiopure C2-symmetric secondary amines. The iridium-phosphoramidite complex successfully manages the dual ARAs by using two distinctly different types of amine sources: an inorganic ammonium salt and an organic primary amine.
Abstract
Asymmetric reductive amination (ARA) is one of the most promising methods for the synthesis of chiral amines. Herein we report our efforts on merging two ARA reactions into a single-step transformation. Catalyzed by a complex formed from iridium and a steric hindered phosphoramidite, readily available and inexpensive aromatic ketones initially undergo the first ARA with ammonium acetate to afford primary amines, which serve as the amine sources for the second ARA, and finally provide the enantiopure C2-symmetric secondary amine products. The developed process competently enables the successive coupling of inorganic and organic nitrogen sources with ketones in the same reaction system. The Brønsted acid additive plays multiple roles in this procedure: it accelerates the formation of imine intermediates, minimizes the inhibitory effect of N-containing species on the iridium catalyst, and reduces the primary amine side products.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202112671-sup-0001-misc_information.pdf6.3 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. S. Hayes, Appl. Catal. A 2001, 221, 187–195;
- 1bS. Gomez, J. A. Peters, T. Maschmeyer, Adv. Synth. Catal. 2002, 344, 1037–1057;
- 1cR. P. Tripathi, S. S. Verma, J. Pandey, V. K. Tiwari, Curr. Org. Chem. 2008, 12, 1093–1115;
- 1dA. F. Abdel-Magid, S. J. Mehrman, Org. Process Res. Dev. 2006, 10, 971–1031;
- 1eE. Podyacheva, O. I. Afanasyev, A. A. Tsygankov, M. Makarova, D. Chusov, Synthesis 2019, 51, 2667–2677;
- 1fO. I. Afanasyev, E. Kuchuk, D. L. Usanov, D. Chusov, Chem. Rev. 2019, 119, 11857–11911;
- 1gT. Irrgang, R. Kempe, Chem. Rev. 2020, 120, 9583–9674;
- 1hK. Murugesan, T. Senthamarai, V. G. Chandrashekhar, K. Natte, P. C. J. Kamer, M. Beller, R. V. Jagadeesh, Chem. Soc. Rev. 2020, 49, 6273–6328;
- 1iA. Trowbridge, S. M. Walton, M. J. Gaunt, Chem. Rev. 2020, 120, 2613–2692.
- 2
- 2a Chiral Amine Synthesis: Methods, Developments and Applications (Ed.: T. C. Nugent), Wiley-VCH, Weinheim, 2010;
10.1002/9783527629541 Google Scholar
- 2bT. C. Nugent, M. El-Shazly, Adv. Synth. Catal. 2010, 352, 753–819;
- 2cC. Wang, J. Xiao, Top. Curr. Chem. 2014, 343, 261–282;
- 2dJ. Barrios-Rivera, Y. Xu, M. Wills, V. K. Vyas, Org. Chem. Front. 2020, 7, 3312–3342;
- 2eY. Tian, L. Hu, Y.-Z. Wang, X. Zhang, Q. Yin, Org. Chem. Front. 2021, 8, 2328–2342.
- 3H.-U. Blaser, H.-P. Buser, H.-P. Jalett, B. Pugin, F. Spindler, Synlett 1999, 867–868.
- 4C. K. Savile, J. M. Janey, E. C. Mundorff, J. C. Moore, S. Tam, W. R. Jarvis, J. C. Colbeck, A. Krebber, F. J. Fleitz, J. Brands, P. N. Devine, G. W. Huisman, G. J. Hughes, Science 2010, 329, 305–309.
- 5N. A. Strotman, C. A. Baxter, K. M. J. Brands, E. Cleator, S. W. Krska, R. A. Reamer, D. J. Wallace, T. J. Wright, J. Am. Chem. Soc. 2011, 133, 8362–8371.
- 6
- 6aD. Steinhuebel, Y.-K. Sun, K. Matsumura, N. Sayo, T. Saito, J. Am. Chem. Soc. 2009, 131, 11316–11317;
- 6bP. Mattei, G. Moine, K. Püntener, R. Schmid, Org. Process Res. Dev. 2011, 15, 353–359;
- 6cK. Matsumura, X.-Y. Zhang, K. Hori, T. Murayama, T. Ohmiya, H. Shimizu, T. Saito, N. Sayo, Org. Process Res. Dev. 2011, 15, 1130–1137;
- 6dY. Lou, Y. Hu, J. Lu, F. Guan, G. Gong, Q. Yin, X. Zhang, Angew. Chem. Int. Ed. 2018, 57, 14193–14197; Angew. Chem. 2018, 130, 14389–14393;
- 6eM. Yamada, K. Azuma, M. Yamano, Org. Lett. 2021, 23, 3364–3367;
- 6fA. C. Brewer, J. C. Ruble, H. G. Vandeveer, S. A. Frank, C. R. Nevill, Jr., Org. Process Res. Dev. 2021, 25, 576–582.
- 7
- 7aR. Kadyrov, T. H. Riermeier, Angew. Chem. Int. Ed. 2003, 42, 5472–5474; Angew. Chem. 2003, 115, 5630–5632;
- 7bX. Tan, S. Gao, W. Zeng, S. Xin, Q. Yin, X. Zhang, J. Am. Chem. Soc. 2018, 140, 2024–2027;
- 7cJ. Gallardo-Donaire, M. Hermsen, J. Wysocki, M. Ernst, F. Rominger, O. Trapp, A. S. K. Hashmi, T. Schaub, J. Am. Chem. Soc. 2018, 140, 355–361;
- 7dT. Ghosh, M. Ernst, A. S. K. Hashmi, T. Schaub, Eur. J. Org. Chem. 2020, 4796–4800;
- 7eL. Hu, Y. Zhang, Q. Zhang, Q. Yin, X. Zhang, Angew. Chem. Int. Ed. 2020, 59, 5321–5325; Angew. Chem. 2020, 132, 5359–5363.
- 8
- 8aY. Chi, Y. Zhou, X. Zhang, J. Org. Chem. 2003, 68, 4120–4122;
- 8bL. Rubio-Pérez, F. J. Pérez-Flores, P. Sharma, L. Velasco, A. Cabrera, Org. Lett. 2009, 11, 265–268;
- 8cC. Li, B. Villa-Marcos, J. Xiao, J. Am. Chem. Soc. 2009, 131, 6967–6969;
- 8dS. Zhou, S. Fleischer, H. Jiao, K. Junge, M. Beller, Adv. Synth. Catal. 2014, 356, 3451–3455;
- 8eP. Yang, L. Lim, P. Chuanprasit, H. Hirao, J. Zhou, Angew. Chem. Int. Ed. 2016, 55, 12083–12087; Angew. Chem. 2016, 128, 12262–12266;
- 8fR.-X. Liu, B. Li, J.-K. Han, D.-X. Zhang, M.-Q. Li, L. Yao, W. Zhao, Q.-F. Wang, R. Jiang, H.-F. Nie, Catal. Sci. Technol. 2020, 10, 5448–5452.
- 9
- 9aR. Kadyrov, T. H. Riermeier, U. Dingerdissen, V. Tararov, A. Borner, J. Org. Chem. 2003, 68, 4067–4070;
- 9bH. Huang, X. Liu, L. Zhou, M. Chang, X. Zhang, Angew. Chem. Int. Ed. 2016, 55, 5309–5312; Angew. Chem. 2016, 128, 5395–5398;
- 9cH. Huang, Y. Zhao, Y. Yang, L. Zhou, M. Chang, Org. Lett. 2017, 19, 1942–1945;
- 9dH. Huang, Z. Wu, G. Gao, L. Zhou, M. Chang, Org. Chem. Front. 2017, 4, 1976–1980;
- 9eG. Gao, S. Du, Y. Yang, X. Lei, H. Huang, M. Chang, Molecules 2018, 23, 2207.
- 10Z.-P. Chen, S.-B. Hu, J. Zhou, Y.-G. Zhou, ACS Catal. 2015, 5, 6086–6089.
- 11
- 11aM. Chang, S. Liu, X. Zhang, Org. Lett. 2013, 15, 4354–4357;
- 11bZ.-P. Chen, S.-B. Hu, M.-W. Chen, Y.-G. Zhou, Org. Lett. 2016, 18, 2676–2679.
- 12Z. Wu, S. Du, G. Gao, W. Yang, H. Huang, M. Chang, Chem. Sci. 2019, 10, 4509–4514.
- 13
- 13aA. J. Minnaard, B. L. Feringa, L. Lefort, J. G. de Vries, Acc. Chem. Res. 2007, 40, 1267–1277;
- 13bW. Fu, W. Tang, ACS Catal. 2016, 6, 4814–4858.
- 14“Advances in the Chemistry of Chiral Lithium Amides”: A. Harrison-Marchand, J. Maddaluno in Lithium Compounds in Organic Synthesis (Eds.: R. Luisi, V. Capriati), Wiley-VCH, Weinheim, 2014, p. 463.
- 15
- 15aT. C. Nugent, A. K. Ghosh, V. N. Wakchaure, R. R. Mohantya, Adv. Synth. Catal. 2006, 348, 1289–1299;
- 15bM. T. Reetz, O. Bondarev, Angew. Chem. Int. Ed. 2007, 46, 4523–4526; Angew. Chem. 2007, 119, 4607–4610;
- 15cS. Guizzetti, M. Benaglia, S. Rossi, Org. Lett. 2009, 11, 2928–2931;
- 15dN. Levi, R. Neumann, ACS Catal. 2013, 3, 1915–1918.
- 16V. N. Wakchaure, B. List, Angew. Chem. Int. Ed. 2016, 55, 15775–15778; Angew. Chem. 2016, 128, 16007–16010.
- 17B. Mitschke, M. Turberg, B. List, Chem 2020, 6, 2515–2532.
- 18For selected examples and reviews of iridium catalysis, see
- 18aG. E. Dobereiner, A. Nova, N. D. Schley, N. Hazari, S. J. Miller, O. Eisenstein, R. H. Crabtree, J. Am. Chem. Soc. 2011, 133, 7547–7562;
- 18bM.-L. Li, S. Yang, X.-C. Su, H.-L. Wu, L.-L. Yang, S.-F. Zhu, Q.-L. Zhou, J. Am. Chem. Soc. 2017, 139, 541–547;
- 18cB. Qu, H. P. R. Mangunuru, S. Tcyrulnikov, D. Rivalti, O. V. Zatolochnaya, D. Kurouski, S. Radomkit, S. Biswas, S. Karyakarte, K. R. Fandrick, J. D. Sieber, S. Rodriguez, J.-N. Desrosiers, N. Haddad, K. McKellop, S. Pennino, H. Lee, N. K. Yee, J. J. Song, M. C. Kozlowski, C. H. Senanayake, Org. Lett. 2018, 20, 1333–1337;
- 18dC.-X. Cui, H. Chen, S.-J. Li, T. Zhang, L.-B. Qu, Y. Lan, Coord. Chem. Rev. 2020, 412, 213251.
- 19
- 19aE. M. Vogl, H. Gröger, M. Shibasaki, Angew. Chem. Int. Ed. 1999, 38, 1570–1577;
10.1002/(SICI)1521-3773(19990601)38:11<1570::AID-ANIE1570>3.0.CO;2-Y CAS PubMed Web of Science® Google ScholarAngew. Chem. 1999, 111, 1672–1680;10.1002/(SICI)1521-3757(19990601)111:11<1672::AID-ANGE1672>3.0.CO;2-2 Web of Science® Google Scholar
- 19bL. Hong, W. Sun, D. Yang, G. Li, R. Wang, Chem. Rev. 2016, 116, 4006–4123.
- 20J.-H. Xie, P.-C. Yan, Q.-Q. Zhang, K.-X. Yuan, Q.-L. Zhou, ACS Catal. 2012, 2, 561–564.