The Formal Cross-Coupling of Amines and Carboxylic Acids to Form sp3–sp3 Carbon–Carbon Bonds
Zirong Zhang
Department of Medicinal Chemistry, College of Pharmacy, University of Michigan, 930 N University Ave, Ann Arbor, MI, 48109 USA
Search for more papers by this authorCorresponding Author
Prof. Tim Cernak
Department of Medicinal Chemistry, College of Pharmacy, University of Michigan, 930 N University Ave, Ann Arbor, MI, 48109 USA
Search for more papers by this authorZirong Zhang
Department of Medicinal Chemistry, College of Pharmacy, University of Michigan, 930 N University Ave, Ann Arbor, MI, 48109 USA
Search for more papers by this authorCorresponding Author
Prof. Tim Cernak
Department of Medicinal Chemistry, College of Pharmacy, University of Michigan, 930 N University Ave, Ann Arbor, MI, 48109 USA
Search for more papers by this authorGraphical Abstract
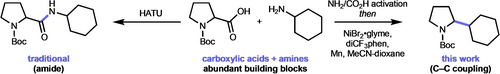
An amine–carboxylic acid C−C coupling would be a valuable addition to the synthetic toolbox of carbon–carbon bond-forming reactions. Using miniaturized high-throughput experimentation, we have developed the first amine–acid cross-coupling to form C(sp3)−C(sp3) bonds based on preactivation of the building blocks and nickel catalysis.
Abstract
We have developed a deaminative–decarboxylative protocol to form new carbon(sp3)–carbon(sp3) bonds from activated amines and carboxylic acids. Amines and carboxylic acids are ubiquitous building blocks, available in broad chemical diversity and at lower cost than typical C−C coupling partners. To leverage amines and acids for C−C coupling, we developed a reductive nickel-catalyzed cross-coupling utilizing building block activation as pyridinium salts and redox-active esters, respectively. Miniaturized high-throughput experimentation studies were critical to our reaction optimization, with subtle experimental changes such as order of reagent addition, composition of a binary solvent system, and ligand identity having a significant impact on reaction performance. The developed protocol is used in the late-stage diversification of pharmaceuticals while more than one thousand systematically captured and machine-readable reaction datapoints are reposited.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202112454-sup-0001-misc_information.pdf10.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1N. Miyaura, K. Yamada, A. Suzuki, Tetrahedron Lett. 1979, 20, 3437–3440.
- 2A. O. King, N. Okukado, E.-I. Negishi, J. Chem. Soc. Chem. Commun. 1977, 683.
- 3J. H. Kirchhoff, M. R. Netherton, I. D. Hills, G. C. Fu, J. Am. Chem. Soc. 2002, 124, 13662–13663.
- 4M. Takeda, K. Nagao, H. Ohmiya, Angew. Chem. Int. Ed. 2020, 59, 22460–22464; Angew. Chem. 2020, 132, 22646–22650.
- 5D. Fernandez Reina, A. Ruffoni, Y. S. S. Al-Faiyz, J. J. Douglas, N. S. Sheikh, D. Leonori, ACS Catal. 2017, 7, 4126–4130.
- 6A. Wilsily, F. Tramutola, N. A. Owston, G. C. Fu, J. Am. Chem. Soc. 2012, 134, 5794–5797.
- 7Z. Lu, G. C. Fu, Angew. Chem. Int. Ed. 2010, 49, 6676–6678; Angew. Chem. 2010, 122, 6826–6828.
- 8B. Saito, G. C. Fu, J. Am. Chem. Soc. 2007, 129, 9602–9603.
- 9M. R. Netherton, C. Dai, K. Neuschütz, G. C. Fu, J. Am. Chem. Soc. 2001, 123, 10099–10100.
- 10H. Huo, B. J. Gorsline, G. C. Fu, Science 2020, 367, 559–564.
- 11X. Mu, Y. Shibata, Y. Makida, G. C. Fu, Angew. Chem. Int. Ed. 2017, 56, 5821–5824; Angew. Chem. 2017, 129, 5915–5918.
- 12J. T. Binder, C. J. Cordier, G. C. Fu, J. Am. Chem. Soc. 2012, 134, 17003–17006.
- 13J. Zhou, G. C. Fu, J. Am. Chem. Soc. 2003, 125, 12527–12530.
- 14R. Giovannini, T. Stüdemann, A. Devasagayaraj, G. Dussin, P. Knochel, J. Org. Chem. 1999, 64, 3544–3553.
- 15Z. Zuo, D. T. Ahneman, L. Chu, J. A. Terrett, A. G. Doyle, D. W. C. Macmillan, Science 2014, 345, 437–440.
- 16C. P. Johnston, R. T. Smith, S. Allmendinger, D. W. C. MacMillan, Nature 2016, 536, 322–325.
- 17Z. Sun, B. Tang, K. K.-C. Liu, H. Y. Zhu, Chem. Commun. 2020, 56, 1294–1297.
- 18J. Cornella, J. T. Edwards, T. Qin, S. Kawamura, J. Wang, C.-M. Pan, R. Gianatassio, M. Schmidt, M. D. Eastgate, P. S. Baran, J. Am. Chem. Soc. 2016, 138, 2174–2177.
- 19L. Huang, A. M. Olivares, D. J. Weix, Angew. Chem. Int. Ed. 2017, 56, 11901–11905; Angew. Chem. 2017, 129, 12063–12067.
- 20T. Qin, J. Cornella, C. Li, L. R. Malins, J. T. Edwards, S. Kawamura, B. D. Maxwell, M. D. Eastgate, P. S. Baran, Science 2016, 352, 801–805.
- 21K. M. M. Huihui, J. A. Caputo, Z. Melchor, A. M. Olivares, A. M. Spiewak, K. A. Johnson, T. A. Dibenedetto, S. Kim, L. K. G. Ackerman, D. J. Weix, J. Am. Chem. Soc. 2016, 138, 5016–5019.
- 22T. Qin, L. R. Malins, J. T. Edwards, R. R. Merchant, A. J. E. Novak, J. Z. Zhong, R. B. Mills, M. Yan, C. Yuan, M. D. Eastgate, P. S. Baran, Angew. Chem. Int. Ed. 2017, 56, 260–265; Angew. Chem. 2017, 129, 266–271.
- 23C. Li, J. Wang, L. M. Barton, S. Yu, M. Tian, D. S. Peters, M. Kumar, A. W. Yu, K. A. Johnson, A. K. Chatterjee, M. Yan, P. S. Baran, Science 2017, 356, eaam7355.
- 24J. Wang, B. P. Cary, P. D. Beyer, S. H. Gellman, D. J. Weix, Angew. Chem. Int. Ed. 2019, 58, 12081–12085; Angew. Chem. 2019, 131, 12209–12213.
- 25X. G. Liu, C. J. Zhou, E. Lin, X. L. Han, S. S. Zhang, Q. Li, H. Wang, Angew. Chem. Int. Ed. 2018, 57, 13096–13100; Angew. Chem. 2018, 130, 13280–13284.
- 26F. Toriyama, J. Cornella, L. Wimmer, T.-G. Chen, D. D. Dixon, G. Creech, P. S. Baran, J. Am. Chem. Soc. 2016, 138, 11132–11135.
- 27J. T. Edwards, R. R. Merchant, K. S. Mcclymont, K. W. Knouse, T. Qin, L. R. Malins, B. Vokits, S. A. Shaw, D.-H. Bao, F.-L. Wei, T. Zhou, M. D. Eastgate, P. S. Baran, Nature 2017, 545, 213–218.
- 28M.-C. Fu, R. Shang, B. Zhao, B. Wang, Y. Fu, Science 2019, 363, 1429–1434.
- 29A. Fawcett, J. Pradeilles, Y. Wang, T. Mutsuga, E. L. Myers, V. K. Aggarwal, Science 2017, 357, 283–286.
- 30R. S. J. Proctor, H. J. Davis, R. J. Phipps, Science 2018, 360, 419–422.
- 31D. Wang, N. Zhu, P. Chen, Z. Lin, G. Liu, J. Am. Chem. Soc. 2017, 139, 15632–15635.
- 32J. Wang, T. Qin, T.-G. Chen, L. Wimmer, J. T. Edwards, J. Cornella, B. Vokits, S. A. Shaw, P. S. Baran, Angew. Chem. Int. Ed. 2016, 55, 9676–9679; Angew. Chem. 2016, 128, 9828–9831.
- 33J. M. Smith, T. Qin, R. R. Merchant, J. T. Edwards, L. R. Malins, Z. Liu, G. Che, Z. Shen, S. A. Shaw, M. D. Eastgate, P. S. Baran, Angew. Chem. Int. Ed. 2017, 56, 11906–11910; Angew. Chem. 2017, 129, 12068–12072.
- 34F. Sandfort, M. J. O'Neill, J. Cornella, L. Wimmer, P. S. Baran, Angew. Chem. Int. Ed. 2017, 56, 3319–3323; Angew. Chem. 2017, 129, 3367–3371.
- 35Z. Li, K.-F. Wang, X. Zhao, H. Ti, X.-G. Liu, H. Wang, Nat. Commun. 2020, 11, 5036.
- 36S. Plunkett, C. H. Basch, S. O. Santana, M. P. Watson, J. Am. Chem. Soc. 2019, 141, 2257–2262.
- 37J. T. M. Correia, V. A. Fernandes, B. T. Matsuo, J. A. C. Delgado, W. C. De Souza, M. W. Paixão, Chem. Commun. 2020, 56, 503–514.
- 38S. Ni, C.-X. Li, Y. Mao, J. Han, Y. Wang, H. Yan, Y. Pan, Sci. Adv. 2019, 5, eaaw9516.
- 39C. H. Basch, J. Liao, J. Xu, J. J. Piane, M. P. Watson, J. Am. Chem. Soc. 2017, 139, 5313–5316.
- 40J. Wu, L. He, A. Noble, V. K. Aggarwal, J. Am. Chem. Soc. 2018, 140, 10700–10704.
- 41F. J. R. Klauck, H. Yoon, M. J. James, M. Lautens, F. Glorius, ACS Catal. 2019, 9, 236–241.
- 42S.-Z. Sun, C. Romano, R. Martin, J. Am. Chem. Soc. 2019, 141, 16197–16201.
- 43D. Kong, P. J. Moon, R. J. Lundgren, Nat. Catal. 2019, 2, 473–476.
- 44J. Wang, M. E. Hoerrner, M. P. Watson, D. J. Weix, Angew. Chem. Int. Ed. 2020, 59, 13484–13489; Angew. Chem. 2020, 132, 13586–13591.
- 45K. M. Baker, D. Lucas Baca, S. Plunkett, M. E. Daneker, M. P. Watson, Org. Lett. 2019, 21, 9738–9741.
- 46M. E. Hoerrner, K. M. Baker, C. H. Basch, E. M. Bampo, M. P. Watson, Org. Lett. 2019, 21, 7356–7360.
- 47J. Liao, C. H. Basch, M. E. Hoerrner, M. R. Talley, B. P. Boscoe, J. W. Tucker, M. R. Garnsey, M. P. Watson, Org. Lett. 2019, 21, 2941–2946.
- 48See ref. [39].
- 49P. Maity, D. M. Shacklady-McAtee, G. P. A. Yap, E. R. Sirianni, M. P. Watson, J. Am. Chem. Soc. 2013, 135, 280–285.
- 50C. J. Helal, M. Bundesmann, S. Hammond, M. Holmstrom, J. Klug-McLeod, B. A. Lefker, D. McLeod, C. Subramanyam, O. Zakaryants, S. Sakata, ACS Med. Chem. Lett. 2019, 10, 1104–1109.
- 51F. W. Goldberg, J. G. Kettle, T. Kogej, M. W. D. Perry, N. P. Tomkinson, Drug Discovery Today 2015, 20, 11–17.
- 52J. Boström, D. G. Brown, R. J. Young, G. M. Keserü, Nat. Rev. Drug Discovery 2018, 17, 709–727.
- 53D. G. Brown, J. Boström, J. Med. Chem. 2016, 59, 4443–4458.
- 54S. D. Roughley, A. M. Jordan, J. Med. Chem. 2011, 54, 3451–3479.
- 55B. Mahjour, Y. Shen, W. Liu, T. Cernak, Nature 2020, 580, 71–75.
- 56See ref. [52].
- 57D. S. Wishart, Y. D. Feunang, A. C. Guo, E. J. Lo, A. Marcu, J. R. Grant, T. Sajed, D. Johnson, C. Li, Z. Sayeeda, N. Assempour, I. Iynkkaran, Y. Liu, A. Maciejewski, N. Gale, A. Wilson, L. Chin, R. Cummings, D. Le, A. Pon, C. Knox, M. Wilson, Nucleic Acids Res. 2018, 46, D1074–D1082.
- 58B. Mahjour, Y. Shen, T. Cernak, Acc. Chem. Res. 2021, 54, 2337–2346.
- 59H. Wong, T. Cernak, Curr. Opin. Green Sustainable Chem. 2018, 11, 91–98.
- 60A. Cook, R. Clément, S. G. Newman, Nat. Protoc. 2021, 16, 1152–1169.
- 61S. W. Krska, D. A. Dirocco, S. D. Dreher, M. Shevlin, Acc. Chem. Res. 2017, 50, 2976–2985.
- 62S. M. Mennen, C. Alhambra, C. L. Allen, M. Barberis, S. Berritt, T. A. Brandt, A. D. Campbell, J. Castañón, A. H. Cherney, M. Christensen, D. B. Damon, J. Eugenio de Diego, S. García-Cerrada, P. García-Losada, R. Haro, J. Janey, D. C. Leitch, L. Li, F. Liu, P. C. Lobben, D. W. C. MacMillan, J. Magano, E. McInturff, S. Monfette, R. J. Post, D. Schultz, B. J. Sitter, J. M. Stevens, I. I. Strambeanu, J. Twilton, K. Wang, M. A. Zajac, Org. Process Res. Dev. 2019, 23, 1213–1242.
- 63T. Cernak, B. Mahjour, ChemRxiv 2020, https://doi.org/10.26434/chemrxiv.13148384.v1.
10.26434/chemrxiv.13148384.v1 Google Scholar
- 64J. Twilton, C. Le, P. Zhang, M. H. Shaw, R. W. Evans, D. W. C. Macmillan, Nat. Rev. Chem. 2017, 1, 0052.
- 65A. R. Katritzky, K. Horvath, B. Plau, J. Chem. Soc. Chem. Commun. 1979, 300.
- 66Y. Shen, J. E. Borowski, M. A. Hardy, R. Sarpong, A. G. Doyle, T. Cernak, Nat. Rev. Methods Primers 2021, 1, 23.
- 67G. Pratsch, G. L. Lackner, L. E. Overman, J. Org. Chem. 2015, 80, 6025–6036.
- 68M. R. Prinsell, D. A. Everson, D. J. Weix, Chem. Commun. 2010, 46, 5743.