Water Splitting by C60-Supported Vanadium Single Atoms
Corresponding Author
Gao-Lei Hou
Quantum Solid-State Physics, Department of Physics and Astronomy, KU Leuven, Celestijnenlaan 200D, 3001 Leuven, Belgium
MOE Key Laboratory for Non-Equilibrium Synthesis and Modulation of Condensed Matter, School of Physics, Xi'an Jiaotong University, Xi'an, 710049 P. R. China
Search for more papers by this authorCorresponding Author
Tao Yang
MOE Key Laboratory for Non-Equilibrium Synthesis and Modulation of Condensed Matter, School of Physics, Xi'an Jiaotong University, Xi'an, 710049 P. R. China
Search for more papers by this authorMengyang Li
MOE Key Laboratory for Non-Equilibrium Synthesis and Modulation of Condensed Matter, School of Physics, Xi'an Jiaotong University, Xi'an, 710049 P. R. China
Search for more papers by this authorJan Vanbuel
Quantum Solid-State Physics, Department of Physics and Astronomy, KU Leuven, Celestijnenlaan 200D, 3001 Leuven, Belgium
Search for more papers by this authorOlga V. Lushchikova
Radboud University, Institute for Molecules and Materials, FELIX Laboratory, Toernooiveld 7, 6525 ED, Nijmegen, The Netherlands
Search for more papers by this authorPiero Ferrari
Quantum Solid-State Physics, Department of Physics and Astronomy, KU Leuven, Celestijnenlaan 200D, 3001 Leuven, Belgium
Search for more papers by this authorJoost M. Bakker
Radboud University, Institute for Molecules and Materials, FELIX Laboratory, Toernooiveld 7, 6525 ED, Nijmegen, The Netherlands
Search for more papers by this authorCorresponding Author
Ewald Janssens
Quantum Solid-State Physics, Department of Physics and Astronomy, KU Leuven, Celestijnenlaan 200D, 3001 Leuven, Belgium
Search for more papers by this authorCorresponding Author
Gao-Lei Hou
Quantum Solid-State Physics, Department of Physics and Astronomy, KU Leuven, Celestijnenlaan 200D, 3001 Leuven, Belgium
MOE Key Laboratory for Non-Equilibrium Synthesis and Modulation of Condensed Matter, School of Physics, Xi'an Jiaotong University, Xi'an, 710049 P. R. China
Search for more papers by this authorCorresponding Author
Tao Yang
MOE Key Laboratory for Non-Equilibrium Synthesis and Modulation of Condensed Matter, School of Physics, Xi'an Jiaotong University, Xi'an, 710049 P. R. China
Search for more papers by this authorMengyang Li
MOE Key Laboratory for Non-Equilibrium Synthesis and Modulation of Condensed Matter, School of Physics, Xi'an Jiaotong University, Xi'an, 710049 P. R. China
Search for more papers by this authorJan Vanbuel
Quantum Solid-State Physics, Department of Physics and Astronomy, KU Leuven, Celestijnenlaan 200D, 3001 Leuven, Belgium
Search for more papers by this authorOlga V. Lushchikova
Radboud University, Institute for Molecules and Materials, FELIX Laboratory, Toernooiveld 7, 6525 ED, Nijmegen, The Netherlands
Search for more papers by this authorPiero Ferrari
Quantum Solid-State Physics, Department of Physics and Astronomy, KU Leuven, Celestijnenlaan 200D, 3001 Leuven, Belgium
Search for more papers by this authorJoost M. Bakker
Radboud University, Institute for Molecules and Materials, FELIX Laboratory, Toernooiveld 7, 6525 ED, Nijmegen, The Netherlands
Search for more papers by this authorCorresponding Author
Ewald Janssens
Quantum Solid-State Physics, Department of Physics and Astronomy, KU Leuven, Celestijnenlaan 200D, 3001 Leuven, Belgium
Search for more papers by this authorGraphical Abstract
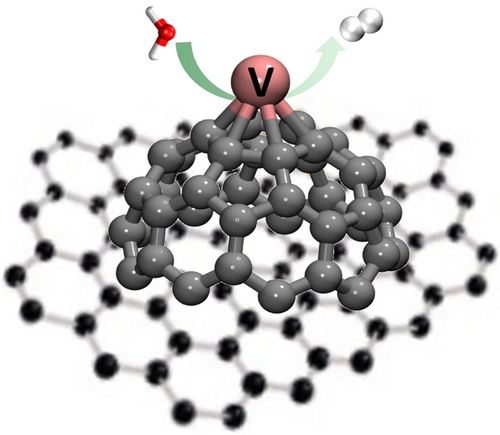
Splitting of water by a single vanadium is greatly facilitated by a C60 support, which can be regarded as a small piece of porous carbon nanomaterials, especially those with intrinsic pentagonal defects and curvatures. The compelling experimental and theoretical evidence demonstrates the important role of support in single vanadium atom catalysis.
Abstract
Water splitting is an important source of hydrogen, a promising future carrier for clean and renewable energy. A detailed understanding of the mechanisms of water splitting, catalyzed by supported metal atoms or nanoparticles, is essential to improve the design of efficient catalysts. Here, we report an infrared spectroscopic study of such a water splitting process, assisted by a C60 supported vanadium atom, C60V++H2O→C60VO++H2. We probe both the entrance channel complex C60V+(H2O) and the end product C60VO+, and observe the formation of H2 as a result from resonant infrared absorption. Density functional theory calculations exploring the detailed reaction pathway reveal that a quintet-to-triplet spin crossing facilitates the water splitting reaction by C60-supported V+, whereas this reaction is kinetically hindered on the isolated V+ ion by a high energy barrier. The C60 support has an important role in lowering the reaction barrier with more than 70 kJ mol−1 due to a large orbital overlap of one water hydrogen atom with one carbon atom of the C60 support. This fundamental insight in the water splitting reaction by a C60-supported single vanadium atom showcases the importance of supports in single atom catalysts by modifying the reaction potential energy surface.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202112398-sup-0001-misc_information.pdf1.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Yang, A. Sudik, C. Wolverton, D. J. Siegel, Chem. Soc. Rev. 2010, 39, 656–675;
- 1bJ. Vanbuel, P. Ferrari, E. Janssens, Adv. Phys. X 2020, 5, 1754132.
- 2J. D. Blakemore, R. H. Crabtree, G. W. Brudvig, Chem. Rev. 2015, 115, 12974–13005.
- 3
- 3aX. Zou, Y. Zhang, Chem. Sco. Rev. 2015, 44, 5148–5180;
- 3bM. M. Najafpour, G. Renger, M. Hołyńska, A. N. Moghaddam, E.-M. Aro, R. Carpentier, H. Nishihara, J. J. Eaton-Rye, J.-R. Shen, S. I. Allakhverdiev, Chem. Rev. 2016, 116, 2886–2936.
- 4
- 4aX.-F. Yang, A. Wang, B. Qiao, J. Li, J. Liu, T. Zhang, Acc. Chem. Res. 2013, 46, 1740–1748;
- 4bJ. Liu, ACS Catal. 2017, 7, 34–59;
- 4cJ.-C. Liu, Y. Tang, Y.-G. Wang, T. Zhang, J. Li, Natl. Sci. Rev. 2018, 5, 638–641;
- 4dH.-Y. Zhuo, X. Zhang, J.-X. Liang, Q. Yu, H. Xiao, J. Li, Chem. Rev. 2020, 120, 12315–12341;
- 4eS. K. Kaiser, Z. Chen, D. F. Akl, S. Mitchell, J. Pérez-Ramírez, Chem. Rev. 2020, 120, 11703–11809;
- 4fR. Lang, X. Du, Y. Huang, X. Jiang, Q. Zhang, Y. Guo, K. Liu, B. Qiao, A. Wang, T. Zhang, Chem. Rev. 2020, 120, 11986–12043.
- 5B. Qiao, A. Wang, X. Yang, L. F. Allard, Z. Jiang, Y. Cui, J. Liu, J. Li, T. Zhang, Nat. Chem. 2011, 3, 634–641.
- 6Y.-G. Wang, D. Mei, V.-A. Glezakou, J. Li, R. Rousseau, Nat. Commun. 2015, 6, 6511.
- 7
- 7aC. Zhu, Q. Shi, S. Feng, D. Du, Y. Lin, ACS Energy Lett. 2018, 3, 1713–1721;
- 7bJ. Deng, H. Li, J. Xiao, Y. Tu, D. Deng, H. Yang, H. Tian, J. Li, P. Ren, X. Bao, Energy Environ. Sci. 2015, 8, 1594–1601;
- 7cC. Ling, L. Shi, Y. Ouyang, X. C. Zeng, J. Wang, Nano Lett. 2017, 17, 5133–5139;
- 7dN. Cheng, S. Stambula, D. Wang, M. N. Banis, J. Liu, A. Riese, B. Xiao, R. Li, T.-K. Sham, L.-M. Liu, G. A. Botton, X. Sun, Nat. Commun. 2016, 7, 13638.
- 8
- 8aK. R. Asmis, A. Fielicke, Top. Catal. 2018, 61, 1–2;
- 8bP. Ferrari, L. M. Molina, V. Kaydashev, J. A. Alonso, P. Lievens, E. Janssens, Angew. Chem. Int. Ed. 2016, 55, 11059–11063; Angew. Chem. 2016, 128, 11225–11229;
- 8cM. Jia, J. Vanbuel, P. Ferrari, E. M. Fernández, S. Gewinner, W. Schöllkopf, M. T. Nguyen, A. Fielicke, E. Janssens, J. Phys. Chem. C 2018, 122, 18247–18255;
- 8dJ. Vanbuel, M.-Y. Jia, P. Ferrari, S. Gewinner, W. Schöllkopf, M. T. Nguyen, A. Fielicke, E. Janssens, Top. Catal. 2018, 61, 62–70;
- 8eY. X. Zhao, Z. Y. Li, Y. Yang, S. G. He, Acc. Chem. Res. 2018, 51, 2603–2610;
- 8fE. Janssens, H. T. Le, P. Lievens, Chem. Eur. J. 2015, 21, 15256–15262;
- 8gH. Zhang, H. Wu, Y. Jia, B. Yin, L. Geng, Z. Luo, K. Hansen, Commun. Chem. 2020, 3, 148;
- 8hY.-X. Zhao, B. Yang, H.-F. Li, Y. Zhang, Y. Yang, Q.-Y. Liu, H.-G. Xu, W.-J. Zheng, S.-G. He, Angew. Chem. Int. Ed. 2020, 59, 21216–21223; Angew. Chem. 2020, 132, 21402–21409;
- 8iG.-L. Hou, E. Faragó, D. Buzsáki, L. Nyulászi, T. Höltzl, E. Janssens, Angew. Chem. Int. Ed. 2021, 60, 4756–4763; Angew. Chem. 2021, 133, 4806–4813;
- 8jH. Zhang, M. Zhang, Y. Jia, L. Geng, B. Yin, S. Li, Z. Luo, F. Pan, J. Phys. Chem. Lett. 2021, 12, 1593–1600.
- 9
- 9aS. M. Lang, I. Fleischer, T. M. Bernhardt, R. N. Barnett, U. Landman, Nano Lett. 2013, 13, 5549–5555;
- 9bS. M. Lang, T. M. Bernhardt, D. M. Kiawi, J. M. Bakker, R. N. Barnett, U. Landman, Angew. Chem. Int. Ed. 2015, 54, 15113–15117; Angew. Chem. 2015, 127, 15328–15332;
- 9cS. M. Lang, T. M. Bernhardt, D. M. Kiawi, J. M. Bakker, R. N. Barnett, U. Landman, Phys. Chem. Chem. Phys. 2016, 18, 15727–15737.
- 10M. R. Fagiani, X. Song, S. Debnath, S. Gewinner, W. Schöllkopf, K. R. Asmis, F. A. Bischoff, F. Muller, J. Sauer, J. Phys. Chem. Lett. 2017, 8, 1272–1277.
- 11G. Liu, E. Miliordos, S. Ciborowski, M. Tschurl, U. Boesl, U. Heiz, X. Zhang, S. Xantheas, K. Bowen, J. Chem. Phys. 2018, 149, 221101.
- 12
- 12aN. R. Walker, R. S. Walters, E. D. Pillai, M. A. Duncan, J. Chem. Phys. 2003, 119, 10471–10474;
- 12bP. D. Carnegie, J. H. Marks, A. D. Brathwaite, T. B. Ward, M. A. Duncan, J. Phys. Chem. A 2020, 124, 1093–1103.
- 13
- 13aD. E. Clemmer, Y.-M. Chen, N. Aristov, P. B. Armentrout, J. Phys. Chem. 1994, 98, 7538–7544;
- 13bA. Irigoras, J. E. Fowler, J. M. Ugalde, J. Am. Chem. Soc. 1999, 121, 574–580;
- 13cY. Xu, Y.-C. Chang, M. Parziale, A. Wannenmacher, C.-Y. Ng, J. Phys. Chem. A 2020, 124, 8884–8896.
- 14B. S. Fox, I. Balteanu, O. P. Balaj, H. Liu, M. K. Beyer, V. E. Bondybey, Phys. Chem. Chem. Phys. 2002, 4, 2224–2228.
- 15
- 15aB. Scharfschwerdt, C. van der Linde, O. P. Balaj, I. Herber, D. Schütze, M. K. Beyer, Low Temp. Phys. 2012, 38, 717–722;
- 15bJ. Heller, T. F. Pascher, D. Muß, C. van der Linde, M. K. Beyer, M. Ončák, Phys. Chem. Chem. Phys. 2021, 23, 22251–22262.
- 16
- 16aJ. Zhu, Y. Huang, W. Mei, C. Zhao, C. Zhang, J. Zhang, I. S. Amiinu, S. Mu, Angew. Chem. Int. Ed. 2019, 58, 3859–3864; Angew. Chem. 2019, 131, 3899–3904;
- 16bM. Chen, S. Wang, H. Zhang, P. Zhang, Z. Tian, M. Lu, X. Xie, L. Huang, W. Huang, Nano Res. 2020, 13, 729–735.
- 17D. M. Merriles, A. Sevy, C. Nielson, M. D. Morse, J. Chem. Phys. 2020, 153, 024303.
- 18
- 18aS. M. Hamilton, W. S. Hopkins, D. J. Harding, T. R. Walsh, P. Gruene, M. Haertelt, A. Fielicke, G. Meijer, S. R. Mackenzie, J. Am. Chem. Soc. 2010, 132, 1448–1449;
- 18bS. M. Hamilton, W. S. Hopkins, D. J. Harding, T. R. Walsh, M. Haertelt, C. Kerpal, P. Gruene, G. Meijer, A. Fielicke, S. R. Mackenzie, J. Phys. Chem. A 2011, 115, 2489–2497;
- 18cI. S. Parry, A. Kartouzian, S. M. Hamilton, O. P. Balaj, M. K. Beyer, S. R. Mackenzie, Angew. Chem. Int. Ed. 2015, 54, 1357–1360; Angew. Chem. 2015, 127, 1373–1377;
- 18dG. Meizyte, A. E. Green, A. S. Gentleman, S. Schaller, W. Schöllkopf, A. Fielicke, S. R. Mackenzie, Phys. Chem. Chem. Phys. 2020, 22, 18606–18613.
- 19H. Schwarz, Int. J. Mass Spectrom. 2004, 237, 75–105.