Enantioselective Palladium-Catalyzed Hydrophosphinylation of Allenes with Phosphine Oxides: Access to Chiral Allylic Phosphine Oxides
Zhiping Yang
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Jun (Joelle) Wang
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Department of Chemistry, Hong Kong Baptist University, Kowloon, Hong Kong, China
Search for more papers by this authorZhiping Yang
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Jun (Joelle) Wang
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Department of Chemistry, Hong Kong Baptist University, Kowloon, Hong Kong, China
Search for more papers by this authorGraphical Abstract
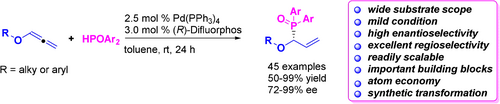
A highly efficient, versatile and atom-economic protocol to chiral allylic phosphine oxides is demonstrated via palladium-catalyzed asymmetric hydrophosphinylation of allenes with phosphine oxides. A family of chiral allylic phosphine oxides with a diverse range of functional groups were obtained in high yield (up to 99 %) and enantioselectivities (up to 99 % ee).
Abstract
A Pd-catalyzed hydrophosphinylation of alkyl and aryl-oxyallenes with phosphine oxides has been developed for the efficient and rapid construction of a family of chiral allylic phosphine oxides with a diverse range of functional groups. This methodology was further applied in the facile construction of chiral 2H-chromene and later stage functionalization of cholesterol.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202112285-sup-0001-misc_information.pdf14.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. P. Horsman, D. L. Zechel, Chem. Rev. 2017, 117, 5704–5783;
- 1bN. J. Niemuth, A. F. Thompson, M. E. Crowe, C. J. Lieven, L. A. Levin, Neurochem. Int. 2016, 99, 24–32;
- 1cW.-S. Huang, S. Liu, D. Zou, M. Thomas, Y. Wang, T. Zhou, J. Romero, A. Kohlmann, F. Li, J. Qi, L. Cai, T. A. Dwight, Y. Xu, R. Xu, R. Dodd, A. Toms, L. Parillon, X. Lu, R. Anjum, S. Zhang, F. Wang, J. Keats, S. D. Wardwell, Y. Ning, Q. Xu, L. E. Moran, Q. K. Mohemmad, H. G. Jang, T. Clackson, N. I. Narasimhan, V. M. Rivera, X. Zhu, D. Dalgarno, W. C. Shakespeare, J. Med. Chem. 2016, 59, 4948–4964;
- 1dR. M. Govea, S. Zhou, S. M. Carlton, Neuroscience 2012, 217, 130–139;
- 1eA. Mucha, P. Kafarski, L. Berlicki, J. Med. Chem. 2011, 54, 5955–5980;
- 1fF. Orsini, G. Sello, M. Sisti, Curr. Med. Chem. 2010, 17, 264–289;
- 1gH. Seto, T. Kuzuyama, Nat. Prod. Rep. 1999, 16, 589–596;
- 1hS. Wu, R. A. Wright, P. K. Rockey, S. G. Burgett, J. S. Arnold, P. R. Rosteck, B. G. Johnson, D. D. Schoepp, R. M. Belagaje, Mol. Brain Res. 1998, 53, 88–97.
- 2
- 2aS. Zhang, D. Yuan, Q. Zhang, Y. Wang, Y. Liu, J. Zhao, B. Chen, J. Mater. Chem. A 2020, 8, 10925–10934;
- 2bH. Kim, J. Lee, H. Jung, J. Porous Mater. 2019, 26, 931–939;
- 2cG. Mallesham, C. Swetha, S. Niveditha, M. E. Mohanty, N. J. Babu, A. Kumar, K. Bhanuprakash, V. J. Rao, J. Mater. Chem. C 2015, 3, 1208–1224;
- 2dS. Gong, Y.-L. Chang, K. Wu, R. White, Z.-H. Lu, D. Song, C. Yang, Chem. Mater. 2014, 26, 1463–1470;
- 2eS. Jin, K. E. Gonsalves, Macromolecules 1998, 31, 1010–1015.
- 3
- 3aH. Ni, W.-L. Chan, Y. Lu, Chem. Rev. 2018, 118, 9344–9411;
- 3bH. Guo, Y. C. Fan, Z. Sun, Y. Wu, O. Kwon, Chem. Rev. 2018, 118, 10049–10293;
- 3cW. Li, J. Zhang, Chem. Soc. Rev. 2016, 45, 1657–1677;
- 3dL.-W. Ye, J. Zhou, Y. Tang, Chem. Soc. Rev. 2008, 37, 1140–1152.
- 4
- 4aS. Zhang, J.-Z. Xiao, Y.-B. Li, C.-Y. Shi, L. Yin, J. Am. Chem. Soc. 2021, 143, 9912–9921;
- 4bC. Wang, K. Huang, J. Ye, W.-L. Duan, J. Am. Chem. Soc. 2021, 143, 5685–5690;
- 4cX.-T. Liu, X.-Y. Han, Y. Wu, Y.-Y. Sun, L. Gao, Z. Huang, Q.-W. Zhang, J. Am. Chem. Soc. 2021, 143, 11309–11316;
- 4dS. Liu, Y. Tanabe, S. Kuriyama, K. Sakata, Y. Nishibayashi, Angew. Chem. Int. Ed. 2021, 60, 11231–11236; Angew. Chem. 2021, 133, 11331–11336;
- 4eQ.-H. Huang, Q.-Y. Zhou, C. Yang, L. Chen, J.-P. Cheng, X. Li, Chem. Sci. 2021, 12, 4582–4587;
- 4fS. Das, Q. Hu, A. Kondoh, M. Terada, Angew. Chem. Int. Ed. 2021, 60, 1417–1422; Angew. Chem. 2021, 133, 1437–1442;
- 4gY.-B. Li, H. Tian, L. Yin, J. Am. Chem. Soc. 2020, 142, 20098–20106;
- 4hQ. Dai, L. Liu, Y. Qian, W. Li, J. Zhang, Angew. Chem. Int. Ed. 2020, 59, 20645–20650; Angew. Chem. 2020, 132, 20826–20831;
- 4iQ. Dai, W. Li, Z. Li, J. Zhang, J. Am. Chem. Soc. 2019, 141, 20556–20564;
- 4jQ. Feng, X. Ma, W. Bao, S.-J. Li, Y. Lan, Q. Song, CCS Chem. 2021, 3, 377–387.
- 5
- 5aG. Li, X. Huo, X. Jiang, W. Zhang, Chem. Soc. Rev. 2020, 49, 2060–2118;
- 5bR. Blieck, M. Taillefer, F. Monnier, Chem. Rev. 2020, 120, 13545–13598;
- 5cD. Berthold, A. G. A. Geissler, S. Giofre, B. Breit, Angew. Chem. Int. Ed. 2019, 58, 9994–9997; Angew. Chem. 2019, 131, 10099–10102;
- 5dZ. Liu, B. Breit, Org. Lett. 2018, 20, 300–303;
- 5eW. Lim, J. Kim, Y. H. Rhee, J. Am. Chem. Soc. 2014, 136, 13618–13621;
- 5fB. M. Trost, J. Xie, J. D. Sieber, J. Am. Chem. Soc. 2011, 133, 20611–20622;
- 5gB. M. Trost, J. Xie, J. Am. Chem. Soc. 2008, 130, 6231–6242;
- 5hB. M. Trost, J. Xie, J. Am. Chem. Soc. 2006, 128, 6044–6045.
- 6
- 6aJ. Zheng, B. Woerl, B. Breit, Eur. J. Org. Chem. 2019, 5180–5182;
- 6bY. Oonishi, A. Hosotani, T. Yokoe, Y. Sato, Org. Lett. 2019, 21, 4120–4123;
- 6cL. J. Hilpert, S. V. Sieger, A. M. Haydl, B. Breit, Angew. Chem. Int. Ed. 2019, 58, 3378–3381; Angew. Chem. 2019, 131, 3416–3419;
- 6dC. P. Grugel, B. Breit, Org. Lett. 2019, 21, 5798–5802;
- 6eP. Steib, B. Breit, Angew. Chem. Int. Ed. 2018, 57, 6572–6576; Angew. Chem. 2018, 130, 6682–6686;
- 6fC. P. Grugel, B. Breit, Org. Lett. 2018, 20, 1066–1069;
- 6gC. P. Grugel, B. Breit, Chem. Eur. J. 2018, 24, 15223–15226;
- 6hP. P. Bora, G.-J. Sun, W.-F. Zheng, Q. Kang, Chin. J. Chem. 2018, 36, 20–24;
- 6iN. Thieme, B. Breit, Angew. Chem. Int. Ed. 2017, 56, 1520–1524; Angew. Chem. 2017, 129, 1542–1546;
- 6jS. Parveen, C. Li, A. Hassan, B. Breit, Org. Lett. 2017, 19, 2326–2329;
- 6kT. M. Beck, B. Breit, Angew. Chem. Int. Ed. 2017, 56, 1903–1907; Angew. Chem. 2017, 129, 1929–1933;
- 6lK. Xu, Y.-H. Wang, V. Khakyzadeh, B. Breit, Chem. Sci. 2016, 7, 3313–3316;
- 6mZ. Liu, B. Breit, Angew. Chem. Int. Ed. 2016, 55, 8440–8443; Angew. Chem. 2016, 128, 8580–8583;
- 6nS. Ganss, B. Breit, Angew. Chem. Int. Ed. 2016, 55, 9738–9742; Angew. Chem. 2016, 128, 9890–9894;
- 6oK. Xu, W. Raimondi, T. Bury, B. Breit, Chem. Commun. 2015, 51, 10861–10863;
- 6pA. B. Pritzius, B. Breit, Angew. Chem. Int. Ed. 2015, 54, 3121–3125; Angew. Chem. 2015, 127, 3164–3168;
- 6qA. B. Pritzius, B. Breit, Angew. Chem. Int. Ed. 2015, 54, 15818–15822; Angew. Chem. 2015, 127, 16044–16048;
- 6rK. Xu, N. Thieme, B. Breit, Angew. Chem. Int. Ed. 2014, 53, 2162–2165; Angew. Chem. 2014, 126, 2194–2197;
- 6sK. Xu, N. Thieme, B. Breit, Angew. Chem. Int. Ed. 2014, 53, 7268–7271; Angew. Chem. 2014, 126, 7396–7399;
- 6tC. Li, M. Kaehny, B. Breit, Angew. Chem. Int. Ed. 2014, 53, 13780–13784; Angew. Chem. 2014, 126, 14000–14004;
- 6uC. Li, B. Breit, J. Am. Chem. Soc. 2014, 136, 862–865;
- 6vM. L. Cooke, K. Xu, B. Breit, Angew. Chem. Int. Ed. 2012, 51, 10876–10879; Angew. Chem. 2012, 124, 11034–11037;
- 6wP. Koschker, A. Lumbroso, B. Breit, J. Am. Chem. Soc. 2011, 133, 20746–20749.
- 7
- 7aD.-J. Jang, S. Lee, J. Lee, D. Moon, Y. H. Rhee, Angew. Chem. Int. Ed. 2021, 60, 22166–22171; Angew. Chem. 2021, 133, 22340–22345;
- 7bZ. Gao, C.-X. Yan, J. Qian, H. Yang, P. Zhou, J. Zhang, G. Jiang, ACS Catal. 2021, 11, 6931–6938;
- 7cS. H. Jang, H. W. Kim, W. Jeong, D. Moon, Y. H. Rhee, Org. Lett. 2018, 20, 1248–1251;
- 7dH. Zhou, Z. Wei, J. Zhang, H. Yang, C. Xia, G. Jiang, Angew. Chem. Int. Ed. 2017, 56, 1077–1081; Angew. Chem. 2017, 129, 1097–1101;
- 7eI. Bernar, B. Fiser, D. Blanco-Ania, E. Gomez-Bengoa, F. P. J. T. Rutjes, Org. Lett. 2017, 19, 4211–4214;
- 7fL. Jiang, T. Jia, M. Wang, J. Liao, P. Cao, Org. Lett. 2015, 17, 1070–1073;
- 7gH. Kim, Y. H. Rhee, J. Am. Chem. Soc. 2012, 134, 4011–4014;
- 7hH. Kim, W. Lim, D. Im, D.-g. Kim, Y. H. Rhee, Angew. Chem. Int. Ed. 2012, 51, 12055–12058; Angew. Chem. 2012, 124, 12221–12224;
- 7iB. M. Trost, A. B. C. Simas, B. Plietker, C. Jakel, J. Xie, Chem. Eur. J. 2005, 11, 7075–7082;
- 7jB. M. Trost, C. Jaekel, B. Plietker, J. Am. Chem. Soc. 2003, 125, 4438–4439.
- 8
- 8aC.-Q. Zhao, L.-B. Han, M. Tanaka, Organometallics 2000, 19, 4196–4198;
- 8bL.-B. Han, F. Mirzaei, C.-Q. Zhao, M. Tanaka, J. Am. Chem. Soc. 2000, 122, 5407–5408.
- 9K. Bravo-Altamirano, I. Abrunhosa-Thomas, J.-L. Montchamp, J. Org. Chem. 2008, 73, 2292–2301.
- 10
- 10aP. Butti, R. Rochat, A. D. Sadow, A. Togni, Angew. Chem. Int. Ed. 2008, 47, 4878–4881; Angew. Chem. 2008, 120, 4956–4959;
- 10bL. Zhang, W. Liu, X. Zhao, Eur. J. Org. Chem. 2014, 6846–6849.
- 11X.-T. Liu, Y.-Q. Zhang, X.-Y. Han, S.-P. Sun, Q.-W. Zhang, J. Am. Chem. Soc. 2019, 141, 16584–16589.
- 12
- 12aW. Sun, L. Hong, C. Liu, R. Wang, Org. Lett. 2010, 12, 3914–3917;
- 12bL. Hong, W. Sun, C. Liu, D. Zhao, R. Wang, Chem. Commun. 2010, 46, 2856–2858.
- 13H. Qiu, Q. Dai, J. He, W. Li, J. Zhang, Chem. Sci. 2020, 11, 9983–9988.
- 14X.-Y. Yang, W. S. Tay, Y. Li, S. A. Pullarkat, P.-H. Leung, Organometallics 2015, 34, 5196–5201.
- 15S.-Z. Nie, R. T. Davison, V. M. Dong, J. Am. Chem. Soc. 2018, 140, 16450–16454.
- 16
- 16aT. Nishimura, S. Hirabayashi, Y. Yasuhara, T. Hayashi, J. Am. Chem. Soc. 2006, 128, 2556–2557;
- 16bT. Nishimura, X.-X. Guo, T. Hayashi, Chem. Asian J. 2008, 3, 1505–1510;
- 16cT. Kawamoto, S. Hirabayashi, X.-X. Guo, T. Nishimura, T. Hayashi, Chem. Commun. 2009, 3528–3530.
- 17
- 17aZ. Yang, X. Gu, L.-B. Han, J. Wang, Chem. Sci. 2020, 11, 7451–7455;
- 17bZ. Lu, H. Zhang, Z. Yang, N. Ding, L. Meng, J. Wang, ACS Catal. 2019, 9, 1457–1463;
- 17cZ. Yang, Q. Du, Y. Jiang, J. Wang, CCS Chem. 2021, 3, 2507–2517;
- 17dL. Meng, K. Y. Ngai, X. Chang, Z. Lin, J. Wang, Org. Lett. 2020, 22, 1155–1159;
- 17eS. Li, Q. Yang, Z. Bian, J. Wang, Org. Lett. 2020, 22, 2781–2785;
- 17fS. Li, Z. Wang, H. Xiao, Z. Bian, J. Wang, Chem. Commun. 2020, 56, 7573–7576;
- 17gQ. Yang, Y. Wang, S. Luo, J. Wang, Angew. Chem. Int. Ed. 2019, 58, 5343–5347; Angew. Chem. 2019, 131, 5397–5401;
- 17hR. Guo, B. Cai, M. Y. Jin, H. Mu, J. Wang, Asian J. Org. Chem. 2019, 8, 687–690;
- 17iS. Luo, L. Meng, Q. Yang, J. Wang, Synlett 2018, 29, 2071–2075;
- 17jJ. Li, Z. Yang, R. Guo, M. Y. Jin, J. Wang, Asian J. Org. Chem. 2018, 7, 1784–1787;
- 17kM. Y. Jin, J. Li, R. Huang, Y. Zhou, L. W. Chung, J. Wang, Chem. Commun. 2018, 54, 4581–4584;
- 17lD. Xiong, W. Zhou, Z. Lu, S. Zeng, J. Wang, Chem. Commun. 2017, 53, 6844–6847;
- 17mL. Meng, M. Y. Jin, J. Wang, Org. Lett. 2016, 18, 4986–4989;
- 17nS. Li, Z. Lu, L. Meng, J. Wang, Org. Lett. 2016, 18, 5276–5279;
- 17oC. Zeng, F. Yang, J. Chen, J. Wang, B. Fan, Org. Biomol. Chem. 2015, 13, 8425–8428;
- 17pQ. Xiao, Q. He, J. Li, J. Wang, Org. Lett. 2015, 17, 6090–6093;
- 17qZ. Lu, J. Wang, B. Han, S. Li, Y. Zhou, B. Fan, Adv. Synth. Catal. 2015, 357, 3121–3125;
- 17rS. Li, J. Xu, B. Fan, Z. Lu, C. Zeng, Z. Bian, Y. Zhou, J. Wang, Chem. Asian J. 2015, 10, 9003–9007;
- 17sQ. He, C. M. So, Z. Bian, T. Hayashi, J. Wang, Chem. Asian J. 2015, 10, 540–543;
- 17tS. Li, H. Chen, Q. Yang, L. Yu, C. Fan, Y. Zhou, J. Wang, B. Fan, Asian J. Org. Chem. 2013, 2, 494–497;
- 17uB. Fan, S. Li, H. Chen, Z. Lu, S. Liu, Q. Yang, L. Yu, J. Xu, Y. Zhou, J. Wang, Adv. Synth. Catal. 2013, 355, 2827–2832.
- 18Deposition Numbers 2107707 (for 3 aa) and 2107709 (for 3 na) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 19L. Ackermann, Synthesis 2006, 1557–1571.
- 20Y. Yamamoto, M. Al-Masum, Synlett 1995, 969–970.