Copper-Catalyzed Difluoromethylation of Alkyl Iodides Enabled by Aryl Radical Activation of Carbon–Iodine Bonds
Dr. Aijie Cai
Department of Chemistry, University of Cincinnati, Cincinnati, OH, 45221 USA
These authors contributed equally to this work.
Search for more papers by this authorWenhao Yan
Department of Chemistry, University of Cincinnati, Cincinnati, OH, 45221 USA
These authors contributed equally to this work.
Search for more papers by this authorChao Wang
Department of Chemistry, University of Cincinnati, Cincinnati, OH, 45221 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Wei Liu
Department of Chemistry, University of Cincinnati, Cincinnati, OH, 45221 USA
Search for more papers by this authorDr. Aijie Cai
Department of Chemistry, University of Cincinnati, Cincinnati, OH, 45221 USA
These authors contributed equally to this work.
Search for more papers by this authorWenhao Yan
Department of Chemistry, University of Cincinnati, Cincinnati, OH, 45221 USA
These authors contributed equally to this work.
Search for more papers by this authorChao Wang
Department of Chemistry, University of Cincinnati, Cincinnati, OH, 45221 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Wei Liu
Department of Chemistry, University of Cincinnati, Cincinnati, OH, 45221 USA
Search for more papers by this authorGraphical Abstract
Abstract
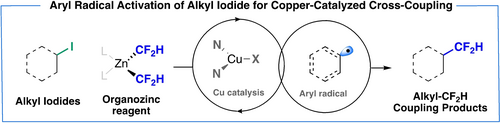
The engagement of unactivated alkyl halides in copper-catalyzed cross-coupling reactions has been historically challenging, due to their low reduction potential and the slow oxidative addition of copper(I) catalysts. In this work, we report a novel strategy that leverages the halogen abstraction ability of aryl radicals, thereby engaging a diverse range of alkyl iodides in copper-catalyzed Negishi-type cross-coupling reactions at room temperature. Specifically, aryl radicals generated via copper catalysis efficiently initiate the cleavage of the carbon–iodide bonds of alkyl iodides. The alkyl radicals thus generated enter the copper catalytic cycles to couple with a difluoromethyl zinc reagent, thus furnishing the alkyl difluoromethane products. This unprecedented Negishi-type difluoromethylation approach has been applied to the late-stage modification of densely functionalized pharmaceutical agents and natural products.
Conflict of interest
A provisional patent has been filed through the University of Cincinnati on methods presented in this paper.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202111993-sup-0001-misc_information.pdf13.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aF. Lovering, MedChemComm 2013, 4, 515–519;
- 1bF. Lovering, J. Bikker, C. Humblet, J. Med. Chem. 2009, 52, 6752–6756.
- 2
- 2aA. de Meijere, S. Bräse, M. Oestreich, Metal-Catalyzed Cross-Coupling Reactions and More, 1, 2 and 3, Wiley-VCH, 2014, 2013;
- 2bF. Diederich, P. J. Stang, Metal-catalyzed Cross-coupling Reactions, Wiley-VCH, New York, 1998;
10.1002/9783527612222 Google Scholar
- 2cJ. Choi, G. C. Fu, Science 2017, 356, eaaf7230;
- 2dA. Rudolph, M. Lautens, Angew. Chem. Int. Ed. 2009, 48, 2656–2670; Angew. Chem. 2009, 121, 2694–2708.
- 3
- 3aN. Hazari, P. R. Melvin, M. M. Beromi, Nat. Rev. Chem. 2017, 1, 0025;
- 3bN. Miyaura, A. Suzuki, Chem. Rev. 1995, 95, 2457–2483;
- 3cA. J. Hickman, M. S. Sanford, Nature 2012, 484, 177—185;
- 3dI. P. Beletskaya, A. V. Cheprakov, Chem. Rev. 2000, 100, 3009–3066.
- 4
- 4aS. Z. Tasker, E. A. Standley, T. F. Jamison, Nature 2014, 509, 299–309;
- 4bJ. C. Tellis, C. B. Kelly, D. N. Primer, M. Jouffroy, N. R. Patel, G. A. Molander, Acc. Chem. Res. 2016, 49, 1429–1439;
- 4cJ. B. Diccianni, T. N. Diao, Trends Chem. 2019, 1, 830–844;
- 4dT. Qin, J. Cornella, C. Li, L. R. Malins, J. T. Edwards, S. Kawamura, B. D. Maxwell, M. D. Eastgate, P. S. Baran, Science 2016, 352, 801–805;
- 4eR. R. Merchant, J. T. Edwards, T. Qin, M. M. Kruszyk, C. Bi, G. Che, D.-H. Bao, W. Qiao, L. Sun, M. R. Collins, O. O. Fadeyi, G. M. Gallego, J. J. Mousseau, P. Nuhant, P. S. Baran, Science 2018, 360, 75–80.
- 5
- 5aN. V. Tsarevsky, K. Matyjaszewski, Chem. Rev. 2007, 107, 2270–2299;
- 5bK. Matyjaszewski, N. V. Tsarevsky, J. Am. Chem. Soc. 2014, 136, 6513–6533;
- 5cS. D. McCann, S. S. Stahl, Acc. Chem. Res. 2015, 48, 1756–1766;
- 5dA. Hossain, A. Bhattacharyya, O. Reiser, Science 2019, 364, eaav9713;
- 5eS. Thapa, B. Shrestha, S. K. Gurung, R. Giri, Org. Biomol. Chem. 2015, 13, 4816–4827;
- 5fR. Trammell, K. Rajabimoghadam, I. Garcia-Bosch, Chem. Rev. 2019, 119, 2954–3031;
- 5gK. S. Egorova, V. P. Ananikov, Angew. Chem. Int. Ed. 2016, 55, 12150–12162; Angew. Chem. 2016, 128, 12334–12347;
- 5hK. S. Egorova, V. P. Ananikov, Organometallics 2017, 36, 4071–4090;
- 5iL.-J. Cheng, N. P. Mankad, Chem. Soc. Rev. 2020, 49, 8036–8064.
- 6sigmaaldrich.com, accessed June 2021.
- 7
- 7aR. A. Festa, D. J. Thiele, Curr. Biol. 2011, 21, R877–R883;
- 7bE. I. Solomon, D. E. Heppner, E. M. Johnston, J. W. Ginsbach, J. Cirera, M. Qayyum, M. T. Kieber-Emmons, C. H. Kjaergaard, R. G. Hadt, L. Tian, Chem. Rev. 2014, 114, 3659–3853;
- 7cE. I. Solomon, R. G. Hadt, Coord. Chem. Rev. 2011, 255, 774–789;
- 7dR. L. Peterson, A. Galaleldeen, J. Villarreal, A. B. Taylor, D. E. Cabelli, P. J. Hart, V. C. Culotta, J. Biol. Chem. 2016, 291, 20911–20923.
- 8“Elemental Impurities in Drug Products Guidance for Industry”, United States Food and Drug Administration, August 2018.
- 9
- 9aC. Chen, J. C. Peters, G. C. Fu, Nature 2021, 596, 250—256;
- 9bS. E. Creutz, K. J. Lotito, G. C. Fu, J. C. Peters, Science 2012, 338, 647;
- 9cA. C. Bissember, R. J. Lundgren, S. E. Creutz, J. C. Peters, G. C. Fu, Angew. Chem. Int. Ed. 2013, 52, 5129–5133; Angew. Chem. 2013, 125, 5233–5237;
- 9dQ. M. Kainz, C. D. Matier, A. Bartoszewicz, S. L. Zultanski, J. C. Peters, G. C. Fu, Science 2016, 351, 681;
- 9eA. Hazra, M. T. Lee, J. F. Chiu, G. Lalic, Angew. Chem. Int. Ed. 2018, 57, 5492–5496; Angew. Chem. 2018, 130, 5590–5594.
- 10
- 10aC. Le, T. Q. Chen, T. Liang, P. Zhang, D. W. C. MacMillan, Science 2018, 360, 1010;
- 10bD. J. P. Kornfilt, D. W. C. MacMillan, J. Am. Chem. Soc. 2019, 141, 6853–6858;
- 10cX. Zhao, D. W. C. MacMillan, J. Am. Chem. Soc. 2020, 142, 19480–19486.
- 11
- 11aB. Górski, A.-L. Barthelemy, J. J. Douglas, F. Juliá, D. Leonori, Nat. Catal. 2021, 4, 623–630;
- 11bT. Constantin, M. Zanini, A. Regni, N. S. Sheikh, F. Juliá, D. Leonori, Science 2020, 367, 1021.
- 12C. Galli, Chem. Rev. 1988, 88, 765–792.
- 13D. Dolenc, B. Plesničar, J. Org. Chem. 2006, 71, 8028–8036.
- 14Y.-R. Luo, Comprehensive handbook of chemical bond energies, CRC Press, Boca Raton, 2007.
10.1201/9781420007282 Google Scholar
- 15M. R. Heinrich, Chem. Eur. J. 2009, 15, 820–833.
- 16
- 16aE. Tatunashvili, C. S. P. McErlean, Org. Biomol. Chem. 2020, 18, 7818–7821;
- 16bA. Chaambi, G. Kurtay, R. Abderrahim, F. Robert, Y. Landais, Helv. Chim. Acta 2019, 102, e1900140;
- 16cD. Kurandina, D. Yadagiri, M. Rivas, A. Kavun, P. Chuentragool, K. Hayama, V. Gevorgyan, J. Am. Chem. Soc. 2019, 141, 8104–8109.
- 17F. Mo, D. Qiu, L. Zhang, J. Wang, Chem. Rev. 2021, 121, 5741–5829.
- 18
- 18aJ. A. Erickson, J. I. McLoughlin, J. Org. Chem. 1995, 60, 1626–1631;
- 18bC. D. Sessler, M. Rahm, S. Becker, J. M. Goldberg, F. Wang, S. J. Lippard, J. Am. Chem. Soc. 2017, 139, 9325–9332;
- 18cD. E. Yerien, S. Barata-Vallejo, A. Postigo, Chem. Eur. J. 2017, 23, 14676–14701;
- 18dJ. Rong, C. Ni, J. Hu, Asian J. Org. Chem. 2017, 6, 139–152;
- 18eY. Lu, C. Liu, Q. Y. Chen, Curr. Org. Chem. 2015, 19, 1638–1650;
- 18fM.-C. Belhomme, T. Besset, T. Poisson, X. Pannecoucke, Chem. Eur. J. 2015, 21, 12836–12865;
- 18gZ. Feng, Y.-L. Xiao, X. Zhang, Acc. Chem. Res. 2018, 51, 2264–2278;
- 18hJ. B. I. Sap, C. F. Meyer, N. J. W. Straathof, N. Iwumene, C. W. am Ende, A. A. Trabanco, V. Gouverneur, Chem. Soc. Rev. 2021, 50, 8214–8247;
- 18iD. R. Carvalho, A. H. Christian, Org. Biomol. Chem. 2021, 19, 947–964.
- 19
- 19aY. Zafrani, G. Sod-Moriah, D. Yeffet, A. Berliner, D. Amir, D. Marciano, S. Elias, S. Katalan, N. Ashkenazi, M. Madmon, E. Gershonov, S. Saphier, J. Med. Chem. 2019, 62, 5628–5637;
- 19bY. Zafrani, D. Yeffet, G. Sod-Moriah, A. Berliner, D. Amir, D. Marciano, E. Gershonov, S. Saphier, J. Med. Chem. 2017, 60, 797–804.
- 20Y. Zafrani, S. Saphier, E. Gershonov, Future Med. Chem. 2020, 12, 361–365.
- 21
- 21aC. Xu, W.-H. Guo, X. He, Y.-L. Guo, X.-Y. Zhang, X. Zhang, Nat. Commun. 2018, 9, 1170;
- 21bL. Xu, D. A. Vicic, J. Am. Chem. Soc. 2016, 138, 2536–2539;
- 21cK. Aikawa, H. Serizawa, K. Ishii, K. Mikami, Org. Lett. 2016, 18, 3690–3693;
- 21dH. Serizawa, K. Ishii, K. Aikawa, K. Mikami, Org. Lett. 2016, 18, 3686–3689;
- 21eC. Lu, Y. Gu, J. Wu, Y. Gu, Q. Shen, Chem. Sci. 2017, 8, 4848–4852;
- 21fY. Gu, X. Leng, Q. Shen, Nat. Commun. 2014, 5, 5405;
- 21gP. S. Fier, J. F. Hartwig, J. Am. Chem. Soc. 2012, 134, 5524–5527;
- 21hV. Bacauanu, S. Cardinal, M. Yamauchi, M. Kondo, D. F. Fernández, R. Remy, D. W. C. MacMillan, Angew. Chem. Int. Ed. 2018, 57, 12543–12548; Angew. Chem. 2018, 130, 12723–12728;
- 21iJ. R. Bour, S. K. Kariofillis, M. S. Sanford, Organometallics 2017, 36, 1220–1223;
- 21jD. Chang, Y. Gu, Q. Shen, Chem. Eur. J. 2015, 21, 6074–6078;
- 21kG. K. S. Prakash, S. K. Ganesh, J.-P. Jones, A. Kulkarni, K. Masood, J. K. Swabeck, G. A. Olah, Angew. Chem. Int. Ed. 2012, 51, 12090–12094; Angew. Chem. 2012, 124, 12256–12260;
- 21lX. L. Jiang, Z. H. Chen, X. H. Xu, F. L. Qing, Org. Chem. Front. 2014, 1, 774–776;
- 21mK. Fujikawa, Y. Fujioka, A. Kobayashi, H. Amii, Org. Lett. 2011, 13, 5560–5563;
- 21nM.-C. Belhomme, T. Poisson, X. Pannecoucke, J. Org. Chem. 2014, 79, 7205–7211.
- 22G. K. S. Prakash, J. Hu, Y. Wang, G. A. Olah, Org. Lett. 2004, 6, 4315–4317.
- 23H. Zhao, C. Lu, S. Herbert, W. Zhang, Q. Shen, J. Org. Chem. 2021, 86, 2854–2865.
- 24S. Monfette, Y.-Q. Fang, M. M. Bio, A. R. Brown, I. T. Crouch, J.-N. Desrosiers, S. Duan, J. M. Hawkins, C. M. Hayward, N. Peperni, J. P. Rainville, Org. Process Res. Dev. 2020, 24, 1077–1083.
- 25
- 25aX. Zeng, W. Yan, S. B. Zacate, A. Cai, Y. Wang, D. Yang, K. Yang, W. Liu, Angew. Chem. Int. Ed. 2020, 59, 16398–16403; Angew. Chem. 2020, 132, 16540–16545;
- 25bX. J. Zeng, W. H. Yan, S. B. Zacate, T. H. Chao, X. D. Sun, Z. Cao, K. G. E. Bradford, M. Paeth, S. B. Tyndall, K. D. Yang, T. C. Kuo, M. J. Cheng, W. Liu, J. Am. Chem. Soc. 2019, 141, 11398–11403;
- 25cX. J. Zeng, W. H. Yang, M. Paeth, S. B. Zacate, P. H. Hong, Y. F. Wang, D. Q. Yang, K. D. Yang, T. Yan, C. Song, Z. Cao, M. J. Cheng, W. Liu, J. Am. Chem. Soc. 2019, 141, 19941–19949.
- 26
- 26aC. Matheis, K. Jouvin, L. J. Goossen, Org. Lett. 2014, 16, 5984–5987;
- 26bA. Cai, W. Yan, X. Zeng, S. B. Zacate, T.-H. Chao, J. A. Krause, M.-J. Cheng, W. Liu, Nat. Commun. 2021, 12, 3272;
- 26cA. Cai, W. Yan, W. Liu, J. Am. Chem. Soc. 2021, 143, 9952–9960.
- 27I. M. DiMucci, J. T. Lukens, S. Chatterjee, K. M. Carsch, C. J. Titus, S. J. Lee, D. Nordlund, T. A. Betley, S. N. MacMillan, K. M. Lancaster, J. Am. Chem. Soc. 2019, 141, 18508–18520.
- 28
- 28aZ. Lu, H. Liu, S. Liu, X. Leng, Y. Lan, Q. Shen, Angew. Chem. Int. Ed. 2019, 58, 8510–8514; Angew. Chem. 2019, 131, 8598–8602;
- 28bS. Liu, H. Liu, S. Liu, Z. Lu, C. Lu, X. Leng, Y. Lan, Q. Shen, J. Am. Chem. Soc. 2020, 142, 9785–9791;
- 28cM. Paeth, S. B. Tyndall, L.-Y. Chen, J.-C. Hong, W. P. Carson, X. Liu, X. Sun, J. Liu, K. Yang, E. M. Hale, D. L. Tierney, B. Liu, Z. Cao, M.-J. Cheng, W. A. Goddard, W. Liu, J. Am. Chem. Soc. 2019, 141, 3153–3159.
- 29
- 29aJ. F. Bunnett, C. C. Wamser, J. Am. Chem. Soc. 1966, 88, 5534–5537;
- 29bD. D. Tanner, D. W. Reed, B. P. Setiloane, J. Am. Chem. Soc. 1982, 104, 3917–3923.
- 30T. Amaya, Y. Jin, M. Tobisu, Tetrahedron Lett. 2019, 60, 151062.
- 31J. C. Scaiano, L. C. Stewart, J. Am. Chem. Soc. 1983, 105, 3609–3614.
- 32
- 32aX.-L. Qiu, X.-H. Xu, F.-L. Qing, Tetrahedron 2010, 66, 789–843;
- 32bA. Cavaliere, K. C. Probst, A. D. Westwell, M. Slusarczyk, Future Med. Chem. 2017, 9, 1809–1833.
- 33P. J. Serafinowski, C. A. Brown, Nucleosides Nucleotides Nucleic Acids 1966, 196, 783.
- 34JAMA 1966, 196, 783–783.
- 35D. J. Portman, G. A. Bachmann, J. A. Simon, Menopause 2013, 20, 623–630.
- 36J. C. Gillis, P. Benfield, D. McTavish, Drugs Aging 1994, 5, 133–152.
- 37G. G. Zhanel, R. Love, H. Adam, A. Golden, S. Zelenitsky, F. Schweizer, B. Gorityala, P. R. S. Lagacé-Wiens, E. Rubinstein, A. Walkty, A. S. Gin, M. Gilmour, D. J. Hoban, J. P. Lynch, J. A. Karlowsky, Drugs 2015, 75, 253–270.
- 38L. Wallentin, R. C. Becker, A. Budaj, C. P. Cannon, H. Emanuelsson, C. Held, J. Horrow, S. Husted, S. James, H. Katus, K. W. Mahaffey, B. M. Scirica, A. Skene, P. G. Steg, R. F. Storey, R. A. Harrington, New Engl. J. Med. 2009, 361, 1045–1057.
- 39N. A. McGrath, M. Brichacek, J. T. Njardarson, J. Chem. Educ. 2010, 87, 1348–1349.