A Unified View on Varied Ultrafast Dynamics of the Primary Process in Microbial Rhodopsins
Dr. Chun-Fu Chang
Molecular Spectroscopy Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama, 351-0198 Japan
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-Ku, Tokyo, 113-0033 Japan
Search for more papers by this authorDr. Hikaru Kuramochi
Molecular Spectroscopy Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama, 351-0198 Japan
Ultrafast Spectroscopy Research Team, RIKEN Center for Advanced Photonics (RAP), RIKEN, 2-1 Hirosawa, Wako, Saitama, 351-0198 Japan
PRESTO (Japan) Science and Technology Agency, 4-1-8 Honcho Kawaguchi, Saitama, 332-0012 Japan
Present address: Research Center of Integrative Molecular Systems, Institute for Molecular Science, 38 Nishigo-Naka, Myodaiji, Okazaki, 444-8585 Japan
Search for more papers by this authorDr. Manish Singh
Department of Life Science and Applied Chemistry, Nagoya Institute of Technology, Showa-Ku, Nagoya, Aichi, 466-8555 Japan
Search for more papers by this authorDr. Rei Abe-Yoshizumi
Department of Life Science and Applied Chemistry, Nagoya Institute of Technology, Showa-Ku, Nagoya, Aichi, 466-8555 Japan
Search for more papers by this authorProf. Tatsuya Tsukuda
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-Ku, Tokyo, 113-0033 Japan
Search for more papers by this authorProf. Hideki Kandori
Department of Life Science and Applied Chemistry, Nagoya Institute of Technology, Showa-Ku, Nagoya, Aichi, 466-8555 Japan
OptoBioTechnology Research Center, Nagoya Institute of Technology, Showa-Ku, Nagoya, Aichi, 466-8555 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Tahei Tahara
Molecular Spectroscopy Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama, 351-0198 Japan
Ultrafast Spectroscopy Research Team, RIKEN Center for Advanced Photonics (RAP), RIKEN, 2-1 Hirosawa, Wako, Saitama, 351-0198 Japan
Search for more papers by this authorDr. Chun-Fu Chang
Molecular Spectroscopy Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama, 351-0198 Japan
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-Ku, Tokyo, 113-0033 Japan
Search for more papers by this authorDr. Hikaru Kuramochi
Molecular Spectroscopy Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama, 351-0198 Japan
Ultrafast Spectroscopy Research Team, RIKEN Center for Advanced Photonics (RAP), RIKEN, 2-1 Hirosawa, Wako, Saitama, 351-0198 Japan
PRESTO (Japan) Science and Technology Agency, 4-1-8 Honcho Kawaguchi, Saitama, 332-0012 Japan
Present address: Research Center of Integrative Molecular Systems, Institute for Molecular Science, 38 Nishigo-Naka, Myodaiji, Okazaki, 444-8585 Japan
Search for more papers by this authorDr. Manish Singh
Department of Life Science and Applied Chemistry, Nagoya Institute of Technology, Showa-Ku, Nagoya, Aichi, 466-8555 Japan
Search for more papers by this authorDr. Rei Abe-Yoshizumi
Department of Life Science and Applied Chemistry, Nagoya Institute of Technology, Showa-Ku, Nagoya, Aichi, 466-8555 Japan
Search for more papers by this authorProf. Tatsuya Tsukuda
Department of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-Ku, Tokyo, 113-0033 Japan
Search for more papers by this authorProf. Hideki Kandori
Department of Life Science and Applied Chemistry, Nagoya Institute of Technology, Showa-Ku, Nagoya, Aichi, 466-8555 Japan
OptoBioTechnology Research Center, Nagoya Institute of Technology, Showa-Ku, Nagoya, Aichi, 466-8555 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Tahei Tahara
Molecular Spectroscopy Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama, 351-0198 Japan
Ultrafast Spectroscopy Research Team, RIKEN Center for Advanced Photonics (RAP), RIKEN, 2-1 Hirosawa, Wako, Saitama, 351-0198 Japan
Search for more papers by this authorGraphical Abstract
Abstract
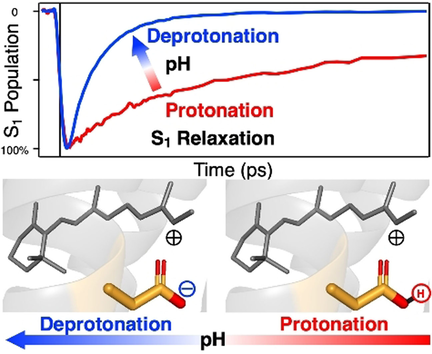
All-trans to 13-cis photoisomerization of the protonated retinal Schiff base (PRSB) chromophore is the primary step that triggers various biological functions of microbial rhodopsins. While this ultrafast primary process has been extensively studied, it has been recognized that the relevant excited-state relaxation dynamics differ significantly from one rhodopsin to another. To elucidate the origin of the complicated ultrafast dynamics of the primary process in microbial rhodopsins, we studied the excited-state dynamics of proteorhodopsin, its D97N mutant, and bacteriorhodopsin by femtosecond time-resolved absorption (TA) spectroscopy in a wide pH range. The TA data showed that their excited-state relaxation dynamics drastically change when pH approaches the pKa of the counterion residue of the PRSB chromophore in the ground state. This result reveals that the varied excited-state relaxation dynamics in different rhodopsins mainly originate from the difference of the ground-state heterogeneity (i.e., protonation/deprotonation of the PRSB counterion).
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202111930-sup-0001-misc_information.pdf3.6 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1O. P. Ernst, D. T. Lodowski, M. Elstner, P. Hegemann, L. S. Brown, H. Kandori, Chem. Rev. 2014, 114, 126–163.
- 2L. A. Gunaydin, O. Yizhar, A. Berndt, V. S. Sohal, K. Deisseroth, P. Hegemann, Nat. Neurosci. 2010, 13, 387–392.
- 3R. A. Mathies, C. H. B. Cruz, W. T. Pollard, C. V. Shank, Science 1988, 240, 777–779.
- 4H. Kandori, K. Yoshihara, H. Tomioka, H. Sasabe, J. Phys. Chem. 1992, 96, 6066–6071.
- 5M. Du, G. R. Fleming, Biophys. Chem. 1993, 48, 101–111.
- 6T. Arlt, S. Schmidt, W. Zinth, U. Haupts, D. Oesterhelt, Chem. Phys. Lett. 1995, 241, 559–565.
- 7S. Ruhman, B. Hou, N. Friedman, M. Ottolenghi, M. Sheves, J. Am. Chem. Soc. 2002, 124, 8854–8858.
- 8J. Herbst, K. Heyne, R. Diller, Science 2002, 297, 822–825.
- 9J. T. M. Kennis, D. S. Larsen, K. Ohta, M. T. Facciotti, R. M. Glaeser, G. R. Fleming, J. Phys. Chem. B 2002, 106, 6067–6080.
- 10B. Schmidt, C. Sobotta, B. Heinz, S. Laimgruber, M. Braun, P. Gilch, Biochim. Biophys. Acta Bioenerg. 2005, 1706, 165–173.
- 11S. Schenkl, F. van Mourik, N. Friedman, M. Sheves, R. Schlesinger, S. Haacke, M. Chergui, Proc. Natl. Acad. Sci. USA 2006, 103, 4101–4106.
- 12D. W. McCamant, P. Kukura, R. A. Mathies, J. Phys. Chem. B 2005, 109, 10449–10457.
- 13M. O. Lenz, R. Huber, B. Schmidt, P. Gilch, R. Kalmbach, M. Engelhard, J. Wachtveitl, Biophys. J. 2006, 91, 255–262.
- 14P. Kukura, D. W. McCamant, R. A. Mathies, Annu. Rev. Phys. Chem. 2007, 58, 461–488.
- 15A. Kahan, O. Nahmias, N. Friedman, M. Sheves, S. Ruhman, J. Am. Chem. Soc. 2007, 129, 537–546.
- 16A. Wand, B. Loevsky, N. Friedman, M. Sheves, S. Ruhman, J. Phys. Chem. B 2013, 117, 4670–4679.
- 17A. Wand, I. Gdor, J. Zhu, M. Sheves, S. Ruhman, Annu. Rev. Phys. Chem. 2013, 64, 437–458.
- 18S. Tahara, S. Takeuchi, R. Abe-Yoshizumi, K. Inoue, H. Ohtani, H. Kandori, T. Tahara, J. Phys. Chem. Lett. 2015, 6, 4481–4486.
- 19E. S. S. Iyer, R. Misra, A. Maity, O. Liubashevski, Y. Sudo, M. Sheves, S. Ruhman, J. Am. Chem. Soc. 2016, 138, 12401–12407.
- 20P. Nogly, T. Weinert, D. James, S. Carbajo, D. Ozerov, A. Furrer, D. Gashi, V. Borin, P. Skopintsev, K. Jaeger, K. Nass, P. Båth, R. Bosman, J. Koglin, M. Seaberg, T. Lane, D. Kekilli, S. Brünle, T. Tanaka, W. Wu, C. Milne, T. White, A. Barty, U. Weierstall, V. Panneels, E. Nango, S. Iwata, M. Hunter, I. Schapiro, G. Schertler, R. Neutze, J. Standfuss, Science 2018, 361, eaat0094.
- 21G. N. Kovacs, J.-P. Colletier, M. L. Grünbein, Y. Yang, T. Stensitzki, A. Batyuk, S. Carbajo, R. B. Doak, D. Ehrenberg, L. Foucar, R. Gasper, A. Gorel, M. Hilpert, M. Kloos, J. E. Koglin, J. Reinstein, C. M. Roome, R. Schlesinger, M. Seaberg, R. L. Shoeman, M. Stricker, S. Boutet, S. Haacke, J. Heberle, K. Heyne, T. Domratcheva, T. R. M. Barends, I. Schlichting, Nat. Commun. 2019, 10, 3177.
- 22S. Tahara, H. Kuramochi, S. Takeuchi, T. Tahara, J. Phys. Chem. Lett. 2019, 10, 5422–5427.
- 23M. C. Nuss, W. Zinth, W. Kaiser, E. Kölling, D. Oesterhelt, Chem. Phys. Lett. 1985, 117, 1–7.
- 24H. J. Polland, M. A. Franz, W. Zinth, W. Kaiser, E. Kölling, D. Oesterhelt, Biophys. J. 1986, 49, 651–662.
- 25F. Peters, J. Herbst, J. Tittor, D. Oesterhelt, R. Diller, Chem. Phys. 2006, 323, 109–116.
- 26R. Huber, T. Köhler, M. O. Lenz, E. Bamberg, R. Kalmbach, M. Engelhard, J. Wachtveitl, Biochemistry 2005, 44, 1800–1806.
- 27J. J. Amsden, J. M. Kralj, L. R. Chieffo, X. Wang, S. Erramilli, E. N. Spudich, J. L. Spudich, L. D. Ziegler, K. J. Rothschild, J. Phys. Chem. B 2007, 111, 11824–11831.
- 28A. Rupenyan, I. H. M. van Stokkum, J. C. Arents, R. van Grondelle, K. Hellingwerf, M. L. Groot, Biophys. J. 2008, 94, 4020–4030.
- 29A. Rupenyan, I. H. M. van Stokkum, J. C. Arents, R. van Grondelle, K. J. Hellingwerf, M. L. Groot, J. Phys. Chem. B 2009, 113, 16251–16256.
- 30R. Rozin, A. Wand, K.-H. Jung, S. Ruhman, M. Sheves, J. Phys. Chem. B 2014, 118, 8995–9006.
- 31M. del C. Marín, D. Agathangelou, Y. Orozco-Gonzalez, A. Valentini, Y. Kato, R. Abe-Yoshizumi, H. Kandori, A. Choi, K.-H. Jung, S. Haacke, M. Olivucci, J. Am. Chem. Soc. 2019, 141, 262–271.
- 32T. Nakamura, S. Takeuchi, M. Shibata, M. Demura, H. Kandori, T. Tahara, J. Phys. Chem. B 2008, 112, 12795–12800.
- 33Y. Sudo, M. Mizuno, Z. Wei, S. Takeuchi, T. Tahara, Y. Mizutani, J. Phys. Chem. B 2014, 118, 1510–1518.
- 34C. Bamann, E. Bamberg, J. Wachtveitl, C. Glaubitz, Biochim. Biophys. Acta Bioenerg. 2014, 1837, 614–625.
- 35S. L. Logunov, M. A. El-Sayed, L. Song, J. K. Lanyi, J. Phys. Chem. 1996, 100, 2391–2398.
- 36F. Gai, K. C. Hasson, J. C. McDonald, P. A. Anfinrud, Science 1998, 279, 1886–1891.
- 37I. Lutz, A. Sieg, A. A. Wegener, M. Engelhard, I. Boche, M. Otsuka, D. Oesterhelt, J. Wachtveitl, W. Zinth, Proc. Natl. Acad. Sci. USA 2001, 98, 962–967.
- 38S. Tahara, S. Takeuchi, R. Abe-Yoshizumi, K. Inoue, H. Ohtani, H. Kandori, T. Tahara, J. Phys. Chem. B 2018, 122, 4784–4792.
- 39H. E. Kato, K. Inoue, R. Abe-Yoshizumi, Y. Kato, H. Ono, M. Konno, S. Hososhima, T. Ishizuka, M. R. Hoque, H. Kunitomo, J. Ito, S. Yoshizawa, K. Yamashita, M. Takemoto, T. Nishizawa, R. Taniguchi, K. Kogure, A. D. Maturana, Y. Iino, H. Yawo, R. Ishitani, H. Kandori, O. Nureki, Nature 2015, 521, 48–53.
- 40C.-F. Chang, H. Kuramochi, M. Singh, R. Abe-Yoshizumi, T. Tsukuda, H. Kandori, T. Tahara, Phys. Chem. Chem. Phys. 2019, 21, 25728–25734.
- 41Y. Sharaabi, V. Brumfeld, M. Sheves, Biochemistry 2010, 49, 4457–4465.
- 42R. Jonas, T. G. Ebrey, Proc. Natl. Acad. Sci. USA 1991, 88, 149–153.
- 43T. Friedrich, S. Geibel, R. Kalmbach, I. Chizhov, K. Ataka, J. Heberle, M. Engelhard, E. Bamberg, J. Mol. Biol. 2002, 321, 821–838.
- 44E. S. Imasheva, S. P. Balashov, J. M. Wang, A. K. Dioumaev, J. K. Lanyi, Biochemistry 2004, 43, 1648–1655.
- 45T. Kouyama, K. Kinosita, A. Ikegami, Biophys. J. 1985, 47, 43–54.
- 46T. Kobayashi, M. Terauchi, T. Kouyama, M. Yoshizawa, M. Taiji, Proc. SPIE-Int. Soc. Opt. Eng. 1990, 1403, 407–416.
- 47L. Song, M. A. El-Sayed, J. K. Lanyi, Science 1993, 261, 891–894.
- 48L. Song, S. L. Logunov, D. Yang, M. A. El-Sayed, Biophys. J. 1994, 67, 2008–2012.
- 49S. L. Logunov, L. Song, M. A. El-Sayed, J. Phys. Chem. 1994, 98, 10674–10677.
- 50K. C. Hasson, F. Gai, P. A. Anfinrud, Proc. Natl. Acad. Sci. USA 1996, 93, 15124–15129.
- 51T. Ye, N. Friedman, Y. Gat, G. H. Atkinson, M. Sheves, M. Ottolenghi, S. Ruhman, J. Phys. Chem. B 1999, 103, 5122–5130.
- 52J. Wang, S. Link, C. D. Heyes, M. A. El-Sayed, Biophys. J. 2002, 83, 1557–1566.
- 53J. Briand, J. Léonard, S. Haacke, J. Opt. 2010, 12, 084004–084015.
- 54A. Wand, N. Friedman, M. Sheves, S. Ruhman, J. Phys. Chem. B 2012, 116, 10444–10452.
- 55K. Tsuji, K. Rosenheck, FEBS Lett. 1979, 98, 368–372.
- 56P. C. Mowery, R. H. Lozier, Q. Chae, Y.-W. Tseng, M. Taylor, W. Stoeckenius, Biochemistry 1979, 18, 4100–4107.
- 57M. O. Lenz, A. C. Woerner, C. Glaubitz, J. Wachtveitl, Photochem. Photobiol. 2007, 83, 226–231.
- 58A. K. Singh, S. M. Sonar, J. Chem. Soc. Perkin Trans. 2 1993, 133.
- 59O. Béjà, L. Aravind, E. V. Koonin, M. T. Suzuki, A. Hadd, L. P. Nguyen, S. B. Jovanovich, C. M. Gates, R. A. Feldman, J. L. Spudich, E. N. Spudich, E. F. DeLong, Science 2000, 289, 1902–1906.
- 60Y. Furutani, D. Ikeda, M. Shibata, H. Kandori, Chem. Phys. 2006, 324, 705–708.
- 61D. Ikeda, Y. Furutani, H. Kandori, Biochemistry 2007, 46, 5365–5373.
- 62H. Okumura, M. Murakami, T. Kouyama, J. Mol. Biol. 2005, 351, 481–495.
- 63H. Abramczyk, J. Chem. Phys. 2004, 120, 11120–11132.
- 64O. Bismuth, P. Komm, N. Friedman, T. Eliash, M. Sheves, S. Ruhman, J. Phys. Chem. B 2010, 114, 3046–3051.
- 65C. Punwong, T. J. Martínez, S. Hannongbua, Chem. Phys. Lett. 2014, 610–611, 213–218.
- 66S. Gozem, H. L. Luk, I. Schapiro, M. Olivucci, Chem. Rev. 2017, 117, 13502–13565.
- 67Y. Guo, F. E. Wolff, I. Schapiro, M. Elstner, M. Marazzi, Phys. Chem. Chem. Phys. 2018, 20, 27501–27509.
- 68V. A. Borin, C. Wiebeler, I. Schapiro, Faraday Discuss. 2018, 207, 137–152.
- 69S. Gozem, P. J. M. Johnson, A. Halpin, H. L. Luk, T. Morizumi, V. I. Prokhorenko, O. P. Ernst, M. Olivucci, R. J. D. Miller, J. Phys. Chem. Lett. 2020, 11, 3889–3896.
- 70F. Hempelmann, S. Hölper, M.-K. Verhoefen, A. C. Woerner, T. Köhler, S.-A. Fiedler, N. Pfleger, J. Wachtveitl, C. Glaubitz, J. Am. Chem. Soc. 2011, 133, 4645–4654.