An Iron(III) Superoxide Corrole from Iron(II) and Dioxygen
Jireh Joy D. Sacramento
Department of Chemistry, The Johns Hopkins University, 3400 North Charles Street, Baltimore, MD, 21218 USA
Search for more papers by this authorTherese Albert
Department of Chemical Physiology and Biochemistry, Oregon Health & Science University, Portland, OR, 97239-3098 USA
Search for more papers by this authorMaxime Siegler
Department of Chemistry, The Johns Hopkins University, 3400 North Charles Street, Baltimore, MD, 21218 USA
Search for more papers by this authorCorresponding Author
Pierre Moënne-Loccoz
Department of Chemical Physiology and Biochemistry, Oregon Health & Science University, Portland, OR, 97239-3098 USA
Search for more papers by this authorCorresponding Author
David P. Goldberg
Department of Chemistry, The Johns Hopkins University, 3400 North Charles Street, Baltimore, MD, 21218 USA
Search for more papers by this authorJireh Joy D. Sacramento
Department of Chemistry, The Johns Hopkins University, 3400 North Charles Street, Baltimore, MD, 21218 USA
Search for more papers by this authorTherese Albert
Department of Chemical Physiology and Biochemistry, Oregon Health & Science University, Portland, OR, 97239-3098 USA
Search for more papers by this authorMaxime Siegler
Department of Chemistry, The Johns Hopkins University, 3400 North Charles Street, Baltimore, MD, 21218 USA
Search for more papers by this authorCorresponding Author
Pierre Moënne-Loccoz
Department of Chemical Physiology and Biochemistry, Oregon Health & Science University, Portland, OR, 97239-3098 USA
Search for more papers by this authorCorresponding Author
David P. Goldberg
Department of Chemistry, The Johns Hopkins University, 3400 North Charles Street, Baltimore, MD, 21218 USA
Search for more papers by this authorGraphical Abstract
Abstract
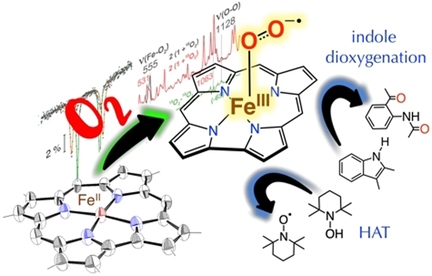
A new structurally characterized ferrous corrole [FeII(ttppc)]− (1) binds one equivalent of dioxygen to form [FeIII(O2−.)(ttppc)]− (2). This complex exhibits a 16/18O2-isotope sensitive ν(O-O) stretch at 1128 cm−1 concomitantly with a single ν(Fe-O2) at 555 cm−1, indicating it is an η1-superoxo (“end-on”) iron(III) complex. Complex 2 is the first well characterized Fe-O2 corrole, and mediates the following biologically relevant oxidation reactions: dioxygenation of an indole derivative, and H-atom abstraction from an activated O−H bond.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202111492-sup-0001-misc_information.pdf8.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aX. Huang, J. T. Groves, Chem. Rev. 2018, 118, 2491–2553;
- 1bJ. B. Gordon, D. P. Goldberg in Comprehensive Coordination Chemistry III, 3rd ed. (Eds.: E. Constable, G. Parkin, L. Que), Elsevier, Amsterdam, 2021, pp. 333–377;
10.1016/B978-0-12-409547-2.14906-6 Google Scholar
- 1cS. Sahu, D. P. Goldberg, J. Am. Chem. Soc. 2016, 138, 11410–11428;
- 1dK. Ray, F. F. Pfaff, B. Wang, W. Nam, J. Am. Chem. Soc. 2014, 136, 13942–13958;
- 1eW. A. van der Donk, C. Krebs, J. M. Bollinger, Jr., Curr. Opin. Struct. Biol. 2010, 20, 673–683.
- 2
- 2aA. Lewis-Ballester, D. Batabyal, T. Egawa, C. Lu, Y. Lin, M. A. Marti, L. Capece, D. A. Estrin, S. R. Yeh, Proc. Natl. Acad. Sci. USA 2009, 106, 17371–17376;
- 2bR. Makino, E. Obayashi, H. Hori, T. Iizuka, K. Mashima, Y. Shiro, Y. Ishimura, Biochemistry 2015, 54, 3604–3616;
- 2cJ. Basran, E. S. Booth, M. Lee, S. Handa, E. L. Raven, Biochemistry 2016, 55, 6743–6750;
- 2dA. Lewis-Ballester, F. Forouhar, S. M. Kim, S. Lew, Y. Wang, S. Karkashon, J. Seetharaman, D. Batabyal, B. Y. Chiang, M. Hussain, M. A. Correia, S. R. Yeh, L. Tong, Sci. Rep. 2016, 6, 35169;
- 2eJ. Geng, A. C. Weitz, K. Dornevil, M. P. Hendrich, A. Liu, Biochemistry 2020, 59, 2813–2822.
- 3Y. Zhang, Y. Zou, N. L. Brock, T. Huang, Y. Lan, X. Wang, Z. Deng, Y. Tang, S. Lin, J. Am. Chem. Soc. 2017, 139, 11887–11894.
- 4Y. Zhu, R. B. Silverman, Biochemistry 2008, 47, 2231–2243.
- 5E. Tamanaha, B. Zhang, Y. Guo, W. C. Chang, E. W. Barr, G. Xing, J. St Clair, S. Ye, F. Neese, J. M. Bollinger, Jr., C. Krebs, J. Am. Chem. Soc. 2016, 138, 8862–8874.
- 6G. Xing, Y. Diao, L. M. Hoffart, E. W. Barr, K. S. Prabhu, R. J. Arner, C. C. Reddy, C. Krebs, J. M. Bollinger, Jr., Proc. Natl. Acad. Sci. USA 2006, 103, 6130–6135.
- 7E. P. Tchesnokov, A. S. Faponle, C. G. Davies, M. G. Quesne, R. Turner, M. Fellner, R. J. Souness, S. M. Wilbanks, S. P. de Visser, G. N. Jameson, Chem. Commun. 2016, 52, 8814–8817.
- 8
- 8aM. L. Pegis, C. F. Wise, D. J. Martin, J. M. Mayer, Chem. Rev. 2018, 118, 2340–2391;
- 8bD. Wang, A. B. Weinstein, P. B. White, S. S. Stahl, Chem. Rev. 2018, 118, 2636–2679;
- 8cH. Sterckx, B. Morel, B. U. W. Maes, Angew. Chem. Int. Ed. 2019, 58, 7946–7970.
- 9
- 9aA. A. Fischer, S. V. Lindeman, A. T. Fiedler, Chem. Commun. 2018, 54, 11344–11347;
- 9bM. N. Blakely, M. A. Dedushko, P. C. Yan Poon, G. Villar-Acevedo, J. A. Kovacs, J. Am. Chem. Soc. 2019, 141, 1867–1870;
- 9cS. Hong, Y.-M. Lee, K. Ray, W. Nam, Coord. Chem. Rev. 2017, 334, 25–42;
- 9dC. Winslow, H. B. Lee, M. J. Field, S. J. Teat, J. Rittle, J. Am. Chem. Soc. 2021, 143, 13686–13693;
- 9eH. R. Pan, H. J. Chen, Z. H. Wu, P. Ge, S. Ye, G. H. Lee, H. F. Hsu, JACS Au 2021, 1, 1389–1398.
- 10
- 10aH. Kim, P. J. Rogler, S. K. Sharma, A. W. Schaefer, E. I. Solomon, K. D. Karlin, J. Am. Chem. Soc. 2020, 142, 3104–3116;
- 10bH. Kim, P. J. Rogler, S. K. Sharma, A. W. Schaefer, E. I. Solomon, K. D. Karlin, Angew. Chem. Int. Ed. 2021, 60, 5907–5912;
- 10cP. Mondal, G. B. Wijeratne, J. Am. Chem. Soc. 2020, 142, 1846–1856;
- 10dP. Mondal, I. Ishigami, E. Gérard, C. Lim, S.-R. Yeh, S. de Visser, G. B. Wijeratne, Chem. Sci. 2021, 12, 8872–8883.
- 11
- 11aS. Mondal, P. K. Naik, J. K. Adha, S. Kar, Coord. Chem. Rev. 2019, 400, 213043–213068;
- 11bJ. J. D. Sacramento, D. P. Goldberg, Acc. Chem. Res. 2018, 51, 2641–2652;
- 11cJ. P. T. Zaragoza, T. H. Yosca, M. A. Siegler, P. Moënne-Loccoz, M. T. Green, D. P. Goldberg, J. Am. Chem. Soc. 2017, 139, 13640–13643;
- 11dJ. P. T. Zaragoza, M. A. Siegler, D. P. Goldberg, J. Am. Chem. Soc. 2018, 140, 4380–4390;
- 11eJ. P. T. Zaragoza, D. C. Cummins, M. Q. E. Mubarak, M. A. Siegler, S. P. de Visser, D. P. Goldberg, Inorg. Chem. 2019, 58, 16761–16770;
- 11fD. C. Cummins, J. G. Alvarado, J. P. T. Zaragoza, M. Q. Effendy Mubarak, Y. T. Lin, S. P. de Visser, D. P. Goldberg, Inorg. Chem. 2020, 59, 16053–16064.
- 12
- 12aK. Oohora, T. Hayashi, Meth. Enzymol. 2016, 580, 439–454;
- 12bT. Matsuo, A. Hayashi, M. Abe, T. Matsuda, Y. Hisaeda, T. Hayashi, J. Am. Chem. Soc. 2009, 131, 15124–15125;
- 12cC. M. Lemon, M. A. Marletta, Inorg. Chem. 2021, 60, 2716–2729.
- 13The UV-vis spectrum for a postulated FeIII(O2)(corrole) was reported: K. P. Caulfield, J. Conradie, H. D. Arman, A. Ghosh, Z. J. Tonzetich, Inorg. Chem. 2019, 58, 15225–15235.
- 14F. Milocco, F. de Vries, H. S. Siebe, S. Engbers, S. Demeshko, F. Meyer, E. Otten, Inorg. Chem. 2021, 60, 2045–2055.
- 15P. D. W. Boyd, D. A. Buckingham, R. F. Mcmeeking, S. Mitra, Inorg. Chem. 1979, 18, 3585–3591.
- 16
- 16aG. Lang, K. Spartalian, C. A. Reed, J. P. Collman, J. Chem. Phys. 1978, 69, 5424–5427;
- 16bJ. P. Collman, J. L. Hoard, N. Kim, G. Lang, C. A. Reed, J. Am. Chem. Soc. 1975, 97, 2676–2681.
- 17
- 17aC. W. Chiang, S. T. Kleespies, H. D. Stout, K. K. Meier, P. Y. Li, E. L. Bominaar, L. Que, Jr., E. Münck, W. Z. Lee, J. Am. Chem. Soc. 2014, 136, 10846–10849;
- 17bM. M. Mbughuni, M. Chakrabarti, J. A. Hayden, E. L. Bominaar, M. P. Hendrich, E. Münck, J. D. Lipscomb, Proc. Natl. Acad. Sci. USA 2010, 107, 16788–16793.
- 18
- 18aM. Schatz, V. Raab, S. P. Foxon, G. Brehm, S. Schneider, M. Reiher, M. C. Holthausen, J. Sundermeyer, S. Schindler, Angew. Chem. Int. Ed. 2004, 43, 4360–4363;
- 18bJ. M. Burke, J. R. Kincaid, S. Peters, R. R. Gagne, J. P. Collman, T. G. Spiro, J. Am. Chem. Soc. 1978, 100, 6083–6088;
- 18cW.-D. Wagner, I. R. Paeng, K. Nakamoto, J. Am. Chem. Soc. 1988, 110, 5565–5567;
- 18dY. Mizutani, S. Hashimoto, Y. Tatsuno, T. Kitagawa, J. Am. Chem. Soc. 1990, 112, 6809–6814;
- 18eL. M. Proniewicz, I. R. Paeng, K. Nakamoto, J. Am. Chem. Soc. 1991, 113, 3294–3303.
- 19K. M. Vogel, P. M. Kozlowski, M. Z. Zgierski, T. G. Spiro, J. Am. Chem. Soc. 1999, 121, 9915–9921.
- 20
- 20aE. Kim, M. E. Helton, I. M. Wasser, K. D. Karlin, S. Lu, H. W. Huang, P. Moënne-Loccoz, C. D. Incarvito, A. L. Rheingold, M. Honecker, S. Kaderli, A. D. Zuberbuhler, Proc. Natl. Acad. Sci. USA 2003, 100, 3623–3628;
- 20bT. K. Das, M. Couture, Y. Ouellet, M. Guertin, D. L. Rousseau, Proc. Natl. Acad. Sci. USA 2001, 98, 479–484.
- 21J. J. D. Sacramento, D. P. Goldberg, Chem. Commun. 2020, 56, 3089–3092.
- 22K. Itakura, K. Uchida, S. Kawakishi, Tetrahedron Lett. 1992, 33, 2567–2570.
- 23E. L. Raven, J. Biol. Inorg. Chem. 2017, 22, 175–183.
- 24J. Y. Lee, R. L. Peterson, K. Ohkubo, I. Garcia-Bosch, R. A. Himes, J. Woertink, C. D. Moore, E. I. Solomon, S. Fukuzumi, K. D. Karlin, J. Am. Chem. Soc. 2014, 136, 9925–9937.
- 25
- 25aJ. J. D. Sacramento, D. P. Goldberg, Chem. Commun. 2019, 55, 913–916;
- 25bM. L. Wind, B. Braun-Cula, F. Schax, C. Herwig, C. Limberg, Isr. J. Chem. 2020, 60, 1057–1060;
- 25cM. Bhadra, W. J. Transue, H. Lim, R. E. Cowley, J. Y. C. Lee, M. A. Siegler, P. Josephs, G. Henkel, M. Lerch, S. Schindler, A. Neuba, K. O. Hodgson, B. Hedman, E. I. Solomon, K. D. Karlin, J. Am. Chem. Soc. 2021, 143, 3707–3713;
- 25dW. D. Bailey, D. Dhar, A. C. Cramblitt, W. B. Tolman, J. Am. Chem. Soc. 2019, 141, 5470–5480;
- 25eS. Hong, K. D. Sutherlin, J. Park, E. Kwon, M. A. Siegler, E. I. Solomon, W. Nam, Nat. Commun. 2014, 5, 5440;
- 25fY. H. Lin, H. H. Cramer, M. van Gastel, Y. H. Tsai, C. Y. Chu, T. S. Kuo, I. R. Lee, S. Ye, E. Bill, W. Z. Lee, Inorg. Chem. 2019, 58, 9756–9765;
- 25gP. C. Duan, D. H. Manz, S. Dechert, S. Demeshko, F. Meyer, J. Am. Chem. Soc. 2018, 140, 4929–4939.
- 26
- 26aJ. B. Gordon, A. C. Vilbert, M. A. Siegler, K. M. Lancaster, P. Moënne-Loccoz, D. P. Goldberg, J. Am. Chem. Soc. 2019, 141, 3641–3653;
- 26bN. Kindermann, C. J. Gunes, S. Dechert, F. Meyer, J. Am. Chem. Soc. 2017, 139, 9831–9834.
- 27Deposition Number 2097620 (for 1) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.