Strongly Coupled Cobalt Diselenide Monolayers for Selective Electrocatalytic Oxygen Reduction to H2O2 under Acidic Conditions
Correction(s) for this article
-
Correction to “Strongly Coupled Cobalt Diselenide Monolayers for Selective Electrocatalytic Oxygen Reduction to H2O2 under Acidic Conditions”
- Volume 64Issue 29Angewandte Chemie International Edition
- First Published online: June 18, 2025
Dr. Xiao-Long Zhang
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorXiaozhi Su
Shanghai Synchrotron Radiation Facility, Shanghai Advanced Research Institute, CAS, Shanghai, 201210 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Ya-Rong Zheng
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Shao-Jin Hu
Division of Theoretical and Computational Sciences, Hefei National Laboratory for Physical Sciences at Microscale, CAS Centre for Excellence and Synergetic Innovation Centre in Quantum Information and Quantum Physics, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Lei Shi
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Fei-Yue Gao
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Peng-Peng Yang
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Zhuang-Zhuang Niu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorZhi-Zheng Wu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorShuai Qin
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Rui Wu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Yu Duan
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Chao Gu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Xu-Sheng Zheng
National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorProf. Jun-Fa Zhu
National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCorresponding Author
Prof. Min-Rui Gao
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Xiao-Long Zhang
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorXiaozhi Su
Shanghai Synchrotron Radiation Facility, Shanghai Advanced Research Institute, CAS, Shanghai, 201210 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Ya-Rong Zheng
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Shao-Jin Hu
Division of Theoretical and Computational Sciences, Hefei National Laboratory for Physical Sciences at Microscale, CAS Centre for Excellence and Synergetic Innovation Centre in Quantum Information and Quantum Physics, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Lei Shi
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Fei-Yue Gao
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Peng-Peng Yang
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Zhuang-Zhuang Niu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorZhi-Zheng Wu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorShuai Qin
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Rui Wu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Yu Duan
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Chao Gu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Xu-Sheng Zheng
National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorProf. Jun-Fa Zhu
National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCorresponding Author
Prof. Min-Rui Gao
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorGraphical Abstract
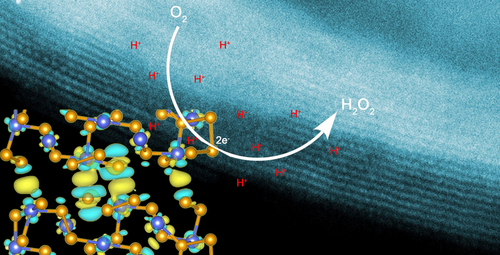
A strategy that narrows the interlayer distance of cobalt diselenide (CoSe2) is reported, which enables strong coupling between CoSe2 monolayers. The strongly coupled CoSe2 can catalyze electrosynthesis of H2O2 in acidic media efficiently, which yields Faradaic efficiency of 96.7 %, current density of 50.04 mA cm−2, and product rate of 30.60 mg cm−2 h−1, outperforming all catalysts reported previously in acidic environments.
Abstract
Electrosynthesis of hydrogen peroxide (H2O2) in the acidic environment could largely prevent its decomposition to water, but efficient catalysts that constitute entirely earth-abundant elements are lacking. Here we report the experimental demonstration of narrowing the interlayer gap of metallic cobalt diselenide (CoSe2), which creates high-performance catalyst to selectively drive two-electron oxygen reduction toward H2O2 in an acidic electrolyte. The enhancement of the interlayer coupling between CoSe2 atomic layers offers a favorable surface electronic structure that weakens the critical *OOH adsorption, promoting the energetics for H2O2 production. Consequently, on the strongly coupled CoSe2 catalyst, we achieved Faradaic efficiency of 96.7 %, current density of 50.04 milliamperes per square centimeter, and product rate of 30.60 mg cm−2 h−1. Moreover, this catalyst shows no sign of degradation when operating at −63 milliamperes per square centimeter over 100 hours.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202111075-sup-0001-SupMat.docx21.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Yang, A. Verdaguer-Casadevall, L. Arnarson, L. Silvioli, V. Čolić, R. Frydendal, J. Rossmeisl, I. Chorkendorff, I. E. Stephens, ACS Catal. 2018, 8, 4064–4081;
- 1bJ. M. Campos-Martin, G. Blanco-Brieva, J. L. G. Fierro, Angew. Chem. Int. Ed. 2006, 45, 6962–6984; Angew. Chem. 2006, 118, 7116–7139;
- 1cE. Brillas, I. Sirés, M. A. Oturan, Chem. Rev. 2009, 109, 6570–6631.
- 2https://www.strategyr.com/market-report-hydrogen-peroxide-forecasts-global-industry-analysts-inc.asp.
- 3
- 3aC. Samanta, Appl. Catal. A 2008, 350, 133–149;
- 3bC. Xia, Y. Xia, P. Zhu, L. Fan, H. Wang, Science 2019, 366, 226–231.
- 4
- 4aY. Jiang, P. Ni, C. Chen, Y. Lu, P. Yang, B. Kong, A. Fisher, X. Wang, Adv. Energy Mater. 2018, 8, 1801909;
- 4bY. Sun, L. Han, P. Strasser, Chem. Soc. Rev. 2020, 49, 6605–6631;
- 4cS. C. Perry, D. Pangotra, L. Vieira, L.-I. Csepei, V. Sieber, L. Wang, C. Ponce de León, F. C. Walsh, Nat. Rev. Chem. 2019, 3, 442–458.
- 5H. W. Kim, M. B. Ross, N. Kornienko, L. Zhang, J. Guo, P. Yang, B. D. McCloskey, Nat. Catal. 2018, 1, 282–290.
- 6Z. Y. Lu, G. X. Chen, S. Siahrostami, Z. H. Chen, K. Liu, J. Xie, L. Liao, T. Wu, D. C. Lin, Y. Y. Liu, T. F. Jaramillo, J. K. Norskov, Y. Cui, Nat. Catal. 2018, 1, 156–162.
- 7K. Jiang, S. Back, A. J. Akey, C. Xia, Y. Hu, W. Liang, D. Schaak, E. Stavitski, J. K. Norskov, S. Siahrostami, H. Wang, Nat. Commun. 2019, 10, 3997.
- 8E. Jung, H. Shin, B.-H. Lee, V. Efremov, S. Lee, H. S. Lee, J. Kim, W. Hooch Antink, S. Park, K.-S. Lee, S.-P. Cho, J. S. Yoo, Y.-E. Sung, T. Hyeon, Nat. Mater. 2020, 19, 436–442.
- 9Z. Qiang, J.-H. Chang, C.-P. Huang, Water Res. 2002, 36, 85–94.
- 10H. F. Mark, J. J. McKetta, D. F. Othmer, Kirk-Othmer encyclopedia of chemical technology, Interscience, New York, 1963.
- 11
- 11aJ. S. Jirkovský, I. Panas, E. Ahlberg, M. Halasa, S. Romani, D. J. Schiffrin, J. Am. Chem. Soc. 2011, 133, 19432–19441;
- 11bA. Verdaguer-Casadevall, D. Deiana, M. Karamad, S. Siahrostami, P. Malacrida, T. W. Hansen, J. Rossmeisl, I. Chorkendorff, I. E. L. Stephens, Nano Lett. 2014, 14, 1603–1608;
- 11cC. H. Choi, M. Kim, H. C. Kwon, S. J. Cho, S. Yun, H. T. Kim, K. J. Mayrhofer, H. Kim, M. Choi, Nat. Commun. 2016, 7, 10922;
- 11dS. Siahrostami, A. Verdaguer-Casadevall, M. Karamad, D. Deiana, P. Malacrida, B. Wickman, M. Escudero-Escribano, E. A. Paoli, R. Frydendal, T. W. Hansen, I. Chorkendorff, I. E. Stephens, J. Rossmeisl, Nat. Mater. 2013, 12, 1137–1143.
- 12
- 12aY. M. Liu, X. Quan, X. F. Fan, H. Wang, S. Chen, Angew. Chem. Int. Ed. 2015, 54, 6837–6841; Angew. Chem. 2015, 127, 6941–6945;
- 12bT. P. Fellinger, F. Hasche, P. Strasser, M. Antonietti, J. Am. Chem. Soc. 2012, 134, 4072–4075.
- 13
- 13aN. Thiyagarajan, D. Janmanchi, Y. F. Tsai, W. H. Wanna, R. Ramu, S. I. Chan, J. M. Zen, S. S. Yu, Angew. Chem. Int. Ed. 2018, 57, 3612–3616; Angew. Chem. 2018, 130, 3674–3678;
- 13bR. B. Valim, R. M. Reis, P. S. Castro, A. S. Lima, R. S. Rocha, M. Bertotti, M. R. Lanza, Carbon 2013, 61, 236–244;
- 13cS. Dey, B. Mondal, S. Chatterjee, A. Rana, S. Amanullah, A. Dey, Nat. Rev. Chem. 2017, 1, 0098.
- 14
- 14aH. Sheng, E. D. Hermes, X. Yang, D. Ying, A. N. Janes, W. Li, J. R. Schmidt, S. Jin, ACS Catal. 2019, 9, 8433–8442;
- 14bH. Y. Sheng, A. N. Janes, R. D. Ross, D. Kaiman, J. Z. Huang, B. Song, J. R. Schmidt, S. Jin, Energy Environ. Sci. 2020, 13, 4189–4203.
- 15C. Xia, S. Back, S. Ringe, K. Jiang, F. Chen, X. Sun, S. Siahrostami, K. Chan, H. Wang, Nat. Catal. 2020, 3, 125–134.
- 16https://www.echemi.com/productsInformation/pid_Rock20578-hydrogenperoxide.html.
- 17D. Iglesias, A. Giuliani, M. Melchionna, S. Marchesan, A. Criado, L. Nasi, M. Bevilacqua, C. Tavagnacco, F. Vizza, M. Prato, Chem 2018, 4, 106–123.
- 18Q. Zhang, X. Tan, N. M. Bedford, Z. Han, L. Thomsen, S. Smith, R. Amal, X. Lu, Nat. Commun. 2020, 11, 4181.
- 19
- 19aY. Sheng, I. A. Abreu, D. E. Cabelli, M. J. Maroney, A.-F. Miller, M. Teixeira, J. S. Valentine, Chem. Rev. 2014, 114, 3854–3918;
- 19bJ. M. McCord, I. Fridovich, J. Biol. Chem. 1969, 244, 6049–6055.
- 20P. Goukec, M. Savy, Electrochim. Acta 1999, 44, 2653–2661.
- 21V. Goellner, V. Armel, A. Zitolo, E. Fonda, F. Jaouen, J. Electrochem. Soc. 2015, 162, H403–H414.
- 22M.-R. Gao, W.-T. Yao, H.-B. Yao, S.-H. Yu, J. Am. Chem. Soc. 2009, 131, 7486–7487.
- 23
- 23aM. R. Gao, Y. F. Xu, J. Jiang, Y. R. Zheng, S. H. Yu, J. Am. Chem. Soc. 2012, 134, 2930–2933;
- 23bX.-L. Zhang, P.-P. Yang, Y.-R. Zheng, Y. Duan, S.-J. Hu, T. Ma, F.-Y. Gao, Z.-Z. Niu, Z.-Z. Wu, S. Qin, L.-P. Chi, X. Yu, R. Wu, C. Gu, C.-M. Wang, X.-S. Zheng, X. Zheng, J.-F. Zhu, M.-R. Gao, Angew. Chem. Int. Ed. 2021, 60, 6553–6560; Angew. Chem. 2021, 133, 6627–6634.
- 24
- 24aX.-L. Zhang, S.-J. Hu, Y.-R. Zheng, R. Wu, F.-Y. Gao, P.-P. Yang, Z.-Z. Niu, C. Gu, X. Yu, X.-S. Zheng, C. Ma, X. Zheng, J.-F. Zhu, M.-R. Gao, S.-H. Yu, Nat. Commun. 2019, 10, 5338;
- 24bY. R. Zheng, P. Wu, M. R. Gao, X. L. Zhang, F. Y. Gao, H. X. Ju, R. Wu, Q. Gao, R. You, W. X. Huang, S. J. Liu, S. W. Hu, J. Zhu, Z. Li, S. H. Yu, Nat. Commun. 2018, 9, 2533.
- 25M. R. Gao, Q. Gao, J. Jiang, C. H. Cui, W. T. Yao, S. H. Yu, Angew. Chem. Int. Ed. 2011, 50, 4905–4908; Angew. Chem. 2011, 123, 5007–5010.
- 26M. Mavrikakis, B. Hammer, J. K. Nørskov, Phys. Rev. Lett. 1998, 81, 2819–2822.
- 27
- 27aN. Ding, M. G. Kanatzidis, Angew. Chem. Int. Ed. 2006, 45, 1397–1401; Angew. Chem. 2006, 118, 1425–1429;
- 27bP. N. Trikalitis, N. Ding, C. Malliakas, S. J. Billinge, M. G. Kanatzidis, J. Am. Chem. Soc. 2004, 126, 15326–15327.
- 28J. M. Charnock, C. M. B. Henderson, J. F. W. M. A. D. Pattrick, Phys. Chem. Miner. 1996, 23, 403–408.
- 29W.-S. Yoon, K.-B. Kim, M.-G. Kim, M.-K. Lee, H.-J. Shin, J.-M. Lee, J.-S. Lee, C.-H. Yo, J. Phys. Chem. B 2002, 106, 2526–2532.
- 30H. Kasai, K. Tolborg, M. Sist, J. Zhang, V. R. Hathwar, M. Ø. Filsø, S. Cenedese, K. Sugimoto, J. Overgaard, E. Nishibori, B. B. Iversen, Nat. Mater. 2018, 17, 249–252.
- 31E. Anastassakis, Solid State Commun. 1973, 13, 1297–1301.
- 32
- 32aL. Samad, S. M. Bladow, Q. Ding, J. Zhuo, R. M. Jacobberger, M. S. Arnold, S. Jin, ACS Nano 2016, 10, 7039–7046;
- 32bX. H. Wang, C. C. Zheng, J. Q. Ning, Sci. Rep. 2016, 6, 33091.
- 33A. J. Bard, L. R. Faulkner, Electrochemical methods: fundamentals and applications, Wiley, New York, 2001.
- 34C. H. Choi, H. C. Kwon, S. Yook, H. Shin, H. Kim, M. Choi, J. Phys. Chem. C 2014, 118, 30063–30070.
- 35R. Shen, W. Chen, Q. Peng, S. Lu, L. Zheng, X. Cao, Y. Wang, W. Zhu, J. Zhang, Z. Zhuang, C. Chen, D. Wang, Y. Li, Chem 2019, 5, 2099–2110.
- 36M. Sano, Inorg. Chem. 1988, 27, 4249–4253.
- 37
- 37aS. W. Lee, S. Chen, J. Suntivich, K. Sasaki, R. R. Adzic, Y. Shao-Horn, J. Phys. Chem. Lett. 2010, 1, 1316–1320;
- 37bX. Zhao, T. Gunji, T. Kaneko, Y. Yoshida, S. Takao, K. Higashi, T. Uruga, W. He, J. Liu, Z. Zou, J. Am. Chem. Soc. 2019, 141, 8516–8526.
- 38E. M. Alexeev, D. A. Ruiz-Tijerina, M. Danovich, M. J. Hamer, D. J. Terry, P. K. Nayak, S. Ahn, S. Pak, J. Lee, J. I. Sohn, M. R. Molas, M. Koperski, K. Watanabe, T. Taniguchi, K. S. Novoselov, R. V. Gorbachev, H. S. Shin, V. I. Fal'ko, A. I. Tartakovskii, Nature 2019, 567, 81–86.