Tailoring the Electronic Structure of an Atomically Dispersed Zinc Electrocatalyst: Coordination Environment Regulation for High Selectivity Oxygen Reduction
Yaling Jia
MOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorDr. Ziqian Xue
Institute for Integrated Cell-Material Sciences (iCeMS), Kyoto University, Kyoto, 606–8501 Japan
These authors contributed equally to this work.
Search for more papers by this authorJun Yang
MOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorQinglin Liu
MOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorJiahui Xian
MOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorYicheng Zhong
MOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorYamei Sun
MOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorXiuxiu Zhang
National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, 230026 P. R. China
Search for more papers by this authorProf. Qinghua Liu
National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, 230026 P. R. China
Search for more papers by this authorProf. Daoxin Yao
State Key Laboratory of Optoelectronic Materials and Technologies, School of Physics, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorCorresponding Author
Prof. Guangqin Li
MOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorYaling Jia
MOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorDr. Ziqian Xue
Institute for Integrated Cell-Material Sciences (iCeMS), Kyoto University, Kyoto, 606–8501 Japan
These authors contributed equally to this work.
Search for more papers by this authorJun Yang
MOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorQinglin Liu
MOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorJiahui Xian
MOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorYicheng Zhong
MOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorYamei Sun
MOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorXiuxiu Zhang
National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, 230026 P. R. China
Search for more papers by this authorProf. Qinghua Liu
National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, 230026 P. R. China
Search for more papers by this authorProf. Daoxin Yao
State Key Laboratory of Optoelectronic Materials and Technologies, School of Physics, Sun Yat-Sen University, Guangzhou, 510275 China
Search for more papers by this authorCorresponding Author
Prof. Guangqin Li
MOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou, 510275 P. R. China
Search for more papers by this authorGraphical Abstract
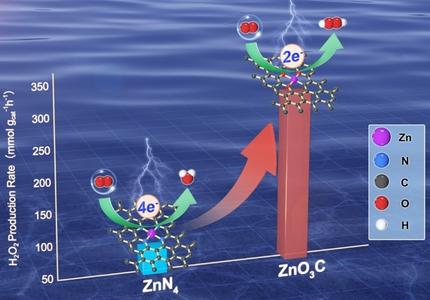
A flexible alteration of an 2 e−/4 e− oxygen reduction reaction (ORR) pathway was discovered by tuning the coordination environment of Zn single sites derived from MOF-5 by varying the functional group of ligands within the metal–organic framework (MOF) precursors. The formed ZnN4 follows a 4 e− ORR pathway, while ZnO3C achieves a maximum H2O2 production rate of 340 mmol g−1 h−1.
Abstract
Accurately regulating the selectivity of the oxygen reduction reaction (ORR) is crucial to renewable energy storage and utilization, but challenging. A flexible alteration of ORR pathways on atomically dispersed Zn sites towards high selectivity ORR can be achieved by tailoring the coordination environment of the catalytic centers. The atomically dispersed Zn catalysts with unique O- and C-coordination structure (ZnO3C) or N-coordination structure (ZnN4) can be prepared by varying the functional groups of corresponding MOF precursors. The coordination environment of as-prepared atomically dispersed Zn catalysts was confirmed by X-ray absorption fine structure (XAFs). Notably, the ZnN4 catalyst processes a 4 e− ORR pathway to generate H2O. However, controllably tailoring the coordination environment of atomically dispersed Zn sites, ZnO3C catalyst processes a 2 e− ORR pathway to generate H2O2 with a near zero overpotential and high selectivity in 0.1 M KOH. Calculations reveal that decreased electron density around Zn in ZnO3C lowers the d-band center of Zn, thus changing the intermediate adsorption and contributing to the high selectivity towards 2 e− ORR.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202110838-sup-0001-misc_information.pdf1.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. Jiang, J. Zhao, H. Wang, Adv. Funct. Mater. 2020, 30, 2003321–2003333;
- 1bK. Chen, K. Liu, P. An, H. Li, Y. Lin, J. Hu, C. Jia, J. Fu, H. Li, H. Liu, Z. Lin, W. Li, J. Li, Y. R. Lu, T. S. Chan, N. Zhang, M. Liu, Nat. Commun. 2020, 11, 4173–4181;
- 1cY. Lin, K. Liu, K. Chen, Y. Xu, H. Li, J. Hu, Y. R. Lu, T. S. Chan, X. Qiu, J. Fu, M. Liu, ACS Catal. 2021, 11, 6304–6315;
- 1dJ. Zhang, Y. Zhao, C. Chen, Y. C. Huang, C. L. Dong, C. J. Chen, R. S. Liu, C. Wang, K. Yan, Y. Li, G. Wang, J. Am. Chem. Soc. 2019, 141, 20118–20126;
- 1eY. Fang, D. Luan, S. Gao, X. W. Lou, Angew. Chem. Int. Ed. 2021, 60, 20102–20118; Angew. Chem. 2021, 133, 20262–20278.
- 2
- 2aG. Wang, J. Chang, S. Koul, A. Kushima, Y. Yang, J. Am. Chem. Soc. 2021, 143, 11595–11601;
- 2bX. F. Lu, B. Y. Xia, S. Q. Zang, X. W. D. Lou, Angew. Chem. Int. Ed. 2020, 59, 4634–4650; Angew. Chem. 2020, 132, 4662–4678;
- 2cL. Jiao, J. Li, L. L. Richard, Q. Sun, T. Stracensky, E. Liu, M. T. Sougrati, Z. Zhao, F. Yang, S. Zhong, H. Xu, S. Mukerjee, Y. Huang, D. A. Cullen, J. H. Park, M. Ferrandon, D. J. Myers, F. Jaouen, Q. Jia, Nat. Mater. 2021, 20, 1385–1391;
- 2dY. Yuan, J. Wang, S. Adimi, H. Shen, T. Thomas, R. Ma, J. P. Attfield, M. Yang, Nat. Mater. 2020, 19, 282–286.
- 3G. Wu, Z. Yang, T. Zhang, Y. Sun, C. Long, Y. Song, S. Lei, Z. Tang, Bull. Korean Chem. Soc. 2021, 42, 1155–1160.
- 4J. Sun, S. E. Lowe, L. Zhang, Y. Wang, K. Pang, Y. Wang, Y. Zhong, P. Liu, K. Zhao, Z. Tang, H. Zhao, Angew. Chem. Int. Ed. 2018, 57, 16511–16515; Angew. Chem. 2018, 130, 16749–16753.
- 5
- 5aZ. Lu, G. Chen, S. Siahrostami, Z. Chen, K. Liu, J. Xie, L. Liao, T. Wu, D. Lin, Y. Liu, T. F. Jaramillo, J. K. Nørskov, Y. Cui, Nat. Catal. 2018, 1, 156–162;
- 5bC. H. Choi, M. Kim, H. C. Kwon, S. J. Cho, S. Yun, H.-T. Kim, K. J. J. Mayrhofer, H. Kim, M. Choi, Nat. Commun. 2016, 7, 10922–10931;
- 5cZ. Zheng, Y. H. Ng, D.-W. Wang, R. Amal, Adv. Mater. 2016, 28, 9949–9955;
- 5dS. Yang, J. Kim, Y. J. Tak, A. Soon, H. Lee, Angew. Chem. Int. Ed. 2016, 55, 2058–2062; Angew. Chem. 2016, 128, 2098–2102;
- 5eH. W. Kim, M. B. Ross, N. Kornienko, L. Zhang, J. Guo, P. Yang, B. D. McCloskey, Nat. Catal. 2018, 1, 282–290.
- 6
- 6aJ. Liu, J. Bak, J. Roh, K.-S. Lee, A. Cho, J. W. Han, E. Cho, ACS Catal. 2021, 11, 466–475;
- 6bG. Chen, P. Liu, Z. Liao, F. Sun, Y. He, H. Zhong, T. Zhang, E. Zschech, M. Chen, G. Wu, J. Zhang, X. Feng, Adv. Mater. 2020, 32, 1907399–1907406;
- 6cX. Li, B. Y. Guan, S. Gao, X. W. Lou, Energy Environ. Sci. 2019, 12, 648–655.
- 7
- 7aN. Zion, D. A. Cullen, P. Zelenay, L. Elbaz, Angew. Chem. Int. Ed. 2020, 59, 2483–2489; Angew. Chem. 2020, 132, 2504–2510;
- 7bZ. Liang, H. Guo, G. Zhou, K. Guo, B. Wang, H. Lei, W. Zhang, H. Zheng, U.-P. Apfel, R. Cao, Angew. Chem. Int. Ed. 2021, 60, 8472–8476; Angew. Chem. 2021, 133, 8553–8557;
- 7cK. Takeyasu, M. Furukawa, Y. Shimoyama, S. K. Singh, J. Nakamura, Angew. Chem. Int. Ed. 2021, 60, 5121–5124; Angew. Chem. 2021, 133, 5181–5184;
- 7dJ. Xie, B.-Q. Li, H.-J. Peng, Y. W. Song, J. X. Li, Z. W. Zhang, Q. Zhang, Angew. Chem. Int. Ed. 2019, 58, 4963–4967; Angew. Chem. 2019, 131, 5017–5021;
- 7eY. Zuo, T. Li, N. Zhang, T. Jing, D. Rao, P. Schmuki, Š. Kment, R. Zbořil, Y. Chai, ACS Nano 2021, 15, 7790–7798.
- 8
- 8aC. Tang, L. Chen, H. Li, L. Li, Y. Jiao, Y. Zheng, H. Xu, K. Davey, S.-Z. Qiao, J. Am. Chem. Soc. 2021, 143, 7819–7827;
- 8bC. Tang, Y. Jiao, B. Shi, J. N. Liu, Z. Xie, X. Chen, Q. Zhang, S. Z. Qiao, Angew. Chem. Int. Ed. 2020, 59, 9171–9176; Angew. Chem. 2020, 132, 9256–9261;
- 8cX. Guo, S. Lin, J. Gu, S. Zhang, Z. Chen, S. Huang, ACS Catal. 2019, 9, 11042–11054.
- 9C. Xia, Y. Xia, P. Zhu, L. Fan, H. Wang, Science 2019, 366, 226–231.
- 10
- 10aA. Kulkarni, S. Siahrostami, A. Patel, J. K. Nørskov, Chem. Rev. 2018, 118, 2302–2312;
- 10bD. San Roman, D. Krishnamurthy, R. Garg, H. Hafiz, M. Lamparski, N. T. Nuhfer, V. Meunier, V. Viswanathan, T. Cohen-Karni, ACS Catal. 2020, 10, 1993–2008.
- 11L. Zou, Y.-S. Wei, C.-C. Hou, C. Li, Q. Xu, Small 2021, 17, 2004809–2004838.
- 12
- 12aX. Liu, L. Zheng, C. Han, H. Zong, G. Yang, S. Lin, A. Kumar, A. R. Jadhav, N. Q. Tran, Y. Hwang, J. Lee, S. Vasimalla, Z. Chen, S.-G. Kim, H. Lee, Adv. Funct. Mater. 2021, 31, 2100547–2100556;
- 12bC. Wang, K. Wang, Y. Feng, C. Li, X. Zhou, L. Gan, Y. Feng, H. Zhou, B. Zhang, X. Qu, H. Li, J. Li, A. Li, Y. Sun, S. Zhang, G. Yang, Y. Guo, S. Yang, T. Zhou, F. Dong, K. Zheng, L. Wang, J. Huang, Z. Zhang, X. Han, Adv. Mater. 2021, 33, 2003327–2003336;
- 12cJ. Xi, H. S. Jung, Y. Xu, F. Xiao, J. W. Bae, S. Wang, Adv. Funct. Mater. 2021, 31, 2008318–2008357;
- 12dY. Lu, T. Liu, C.-L. Dong, Y. C. Huang, Y. Li, J. Chen, Y. Zou, S. Wang, Adv. Mater. 2021, 33, 2007056–2007062.
- 13
- 13aY. Zhou, X. Tao, G. Chen, R. Lu, D. Wang, M. X. Chen, E. Jin, J. Yang, H. W. Liang, Y. Zhao, X. Feng, A. Narita, K. Mullen, Nat. Commun. 2020, 11, 5892–5903;
- 13bE. Jung, H. Shin, B.-H. Lee, V. Efremov, S. Lee, H. S. Lee, J. Kim, W. Hooch Antink, S. Park, K.-S. Lee, S.-P. Cho, J. S. Yoo, Y.-E. Sung, T. Hyeon, Nat. Mater. 2020, 19, 436–442;
- 13cJ. Wan, Z. Zhao, H. Shang, B. Peng, W. Chen, J. Pei, L. Zheng, J. Dong, R. Cao, R. Sarangi, Z. Jiang, D. Zhou, Z. Zhuang, J. Zhang, D. Wang, Y. Li, J. Am. Chem. Soc. 2020, 142, 8431–8439.
- 14
- 14aL. Jiao, H.-L. Jiang, Chem 2019, 5, 786–804;
- 14bH. Xiang, W. Feng, Y. Chen, Adv. Mater. 2020, 32, 1905994;
- 14cY. Jiao, Y. Zheng, P. Chen, M. Jaroniec, S. Z. Qiao, J. Am. Chem. Soc. 2017, 139, 18093–18100;
- 14dJ. C. Liu, H. Xiao, J. Li, J. Am. Chem. Soc. 2020, 142, 3375–3383.
- 15
- 15aJ. Liu, D. Zhu, C. Guo, A. Vasileff, S. Z. J. A. E. M. Qiao, Adv. Energy Mater. 2017, 7, 1700518–1700544;
- 15bS. Yang, Y. J. Tak, J. Kim, A. Soon, H. Lee, ACS Catal. 2017, 7, 1301–1307.
- 16
- 16aZ. Xue, K. Liu, Q. Liu, Y. Li, M. Li, C.-Y. Su, N. Ogiwara, H. Kobayashi, H. Kitagawa, M. Liu, G. Li, Nat. Commun. 2019, 10, 5048;
- 16bT. Qiu, Z. Liang, W. Guo, H. Tabassum, S. Gao, R. Zou, ACS Energy Lett. 2020, 5, 520–532;
- 16cZ. Liang, C. Qu, W. Guo, R. Zou, Q. Xu, Adv. Mater. 2018, 30, 1702891.
- 17
- 17aA. Han, B. Wang, A. Kumar, Y. Qin, J. Jin, X. Wang, C. Yang, B. Dong, Y. Jia, J. Liu, X. Sun, Small Methods 2019, 3, 1800471;
- 17bZ. Song, L. Zhang, K. Doyle-Davis, X. Fu, J.-L. Luo, X. Sun, Adv. Energy Mater. 2020, 10, 2001561.
- 18C. S. Tsao, M. S. Yu, T. Y. Chung, H. C. Wu, C. Y. Wang, K. S. Chang, H. L. Chen, J. Am. Chem. Soc. 2007, 129, 15997–16004.
- 19
- 19aY. Sun, L. Silvioli, N. R. Sahraie, W. Ju, J. Li, A. Zitolo, S. Li, A. Bagger, L. Arnarson, X. Wang, T. Moeller, D. Bernsmeier, J. Rossmeisl, F. Jaouen, P. Strasser, J. Am. Chem. Soc. 2019, 141, 12372–12381;
- 19bW. Ju, A. Bagger, G.-P. Hao, A. S. Varela, I. Sinev, V. Bon, B. Roldan Cuenya, S. Kaskel, J. Rossmeisl, P. Strasser, Nat. Commun. 2017, 8, 944.
- 20
- 20aY. Li, B. Jia, Y. Fan, K. Zhu, G. Li, C. Y. Su, Adv. Energy Mater. 2017, 8, 1702048;
- 20bM. S. Dresselhaus, A. Jorio, M. Hofmann, G. Dresselhaus, R. Saito, Nano Lett. 2010, 10, 751–758.
- 21
- 21aP. Song, M. Luo, X. Liu, W. Xing, W. Xu, Z. Jiang, L. Gu, Adv. Funct. Mater. 2017, 27, 1700802;
- 21bM. Uğurlu, A. Gürses, Ç. Doğar, Color. Technol. 2007, 123, 106–114.
- 22L. Han, S. Song, M. Liu, S. Yao, Z. Liang, H. Cheng, Z. Ren, W. Liu, R. Lin, G. Qi, X. Liu, Q. Wu, J. Luo, H. L. Xin, J. Am. Chem. Soc. 2020, 142, 12563–12567.
- 23F. Yang, P. Song, X. Liu, B. Mei, W. Xing, Z. Jiang, L. Gu, W. Xu, Angew. Chem. Int. Ed. 2018, 57, 12303–12307; Angew. Chem. 2018, 130, 12483–12487.
- 24
- 24aJ. Zhao, S. Ji, C. Guo, H. Li, J. Dong, P. Guo, D. Wang, Y. Li, F. D. Toste, Nat. Catal. 2021, 4, 523–531;
- 24bM. Muñoz, F. Farges, P. Argoul, CCWT analyses of XAFS spectra 2003, 2;
- 24cM. Munoz, P. Argoul, F. Farges, Am. Mineral. 2003, 88, 694–700.
- 25Z. Chen, J. Song, X. Peng, S. Xi, J. Liu, W. Zhou, R. Li, R. Ge, C. Liu, H. Xu, X. Zhao, H. Li, X. Zhou, L. Wang, X. Li, L. Zhong, A. I. Rykov, J. Wang, M. J. Koh, K. P. Loh, Adv. Mater. 2021, 2101382.
- 26T. Wang, X. Cao, H. Qin, L. Shang, S. Zheng, F. Fang, L. Jiao, Angew. Chem. Int. Ed. 2021, 60, 21237–21241; Angew. Chem. 2021, 133, 21407–21411.
- 27L. Huang, X. Zheng, G. Gao, H. Zhang, K. Rong, J. Chen, Y. Liu, X. Zhu, W. Wu, Y. Wang, J. Wang, S. Dong, J. Am. Chem. Soc. 2021, 143, 6933–6941.
- 28
- 28aP. Vassilev, M. T. M. Koper, J. Phys. Chem. C 2007, 111, 2607–2613;
- 28bS. Siahrostami, A. Verdaguer-Casadevall, M. Karamad, D. Deiana, P. Malacrida, B. Wickman, M. Escudero-Escribano, E. A. Paoli, R. Frydendal, T. W. Hansen, I. Chorkendorff, I. E. L. Stephens, J. Rossmeisl, Nat. Mater. 2013, 12, 1137–1143.
- 29X. Zhao, Y. Liu, J. Am. Chem. Soc. 2021, 143, 9423–9428.
- 30X. Han, T. Zhang, W. Chen, B. Dong, G. Meng, L. Zheng, C. Yang, X. Sun, Z. Zhuang, D. Wang, A. Han, J. Liu, Adv. Energy Mater. 2021, 11, 2002753.
- 31B. W. Noffke, Q. Li, K. Raghavachari, L. S. Li, J. Am. Chem. Soc. 2016, 138, 13923–13929.